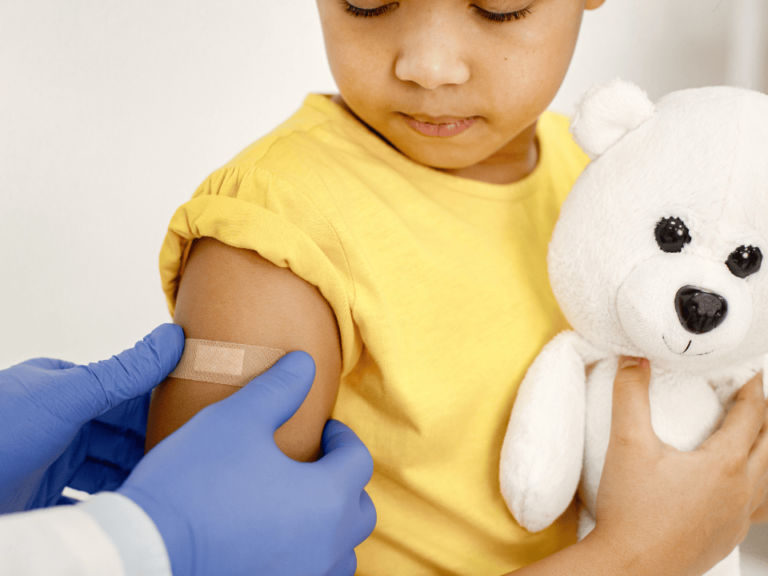
At a two-day meeting, the Center for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted 8-3 to change the current recommendations that allow children under age 4 to receive the MMRV vaccine, a combination shot for measles, mumps, rubella and chickenpox (or varicella).
To access this subscriber-only content please log in or subscribe.
If your institution has a site license, log in with IP-login or register for a sponsored account.*
*Not all site licenses are enrolled in sponsored accounts.
Login Subscribe
If your institution has a site license, log in with IP-login or register for a sponsored account.*
*Not all site licenses are enrolled in sponsored accounts.
Login Subscribe