The Oregon Health Authority did a considerable amount of work to prepare a plan that would deny Medicaid coverage for next-generation sequencing tests in the state.
A review of medical literature it conducted resolved that “direct evidence of clinical utility is not available” for NGS. Then, a guidance was drafted, approved unanimously by an advisory panel, and brought forth to a public hearing Sept. 27.
But there, the proposal encountered stiff opposition from Oregonians, who argued that it would create a massive health disparity in the state. The younger Oregonians eligible for Medicaid would be denied access to precision oncology, while the elderly would continue to get access under Medicare.
“I want to thank everyone who spoke up to make sure that NGS for patients with cancer is covered by Oregon Medicaid,” Brian Druker, director of the Knight Cancer Institute at the Oregon Health & Science University, said to The Cancer Letter. “Your voices were clearly heard and I applaud the committee for ensuring that our most vulnerable patients have access to this cutting edge technology.”
Druker’s institution lobbied heavily to stop the plan. Meanwhile, the chair of the subcommittee that unanimously approved the draft guidance, Vinay Prasad, reports to Druker. In recent months, Prasad, a hematologist-oncologist at OHSU, has emerged as a leading critic of precision oncology.
Insiders say that a Sept. 26 story published in The Cancer Letter was circulated among those alarmed by the Oregon proposal, including those present at the Sept. 27 public hearing.
At the conclusion of the meeting, the Health Technology Assessment Subcommittee, a part of the OHA’s Health Evidence Review Commission, decided to table the draft guidance indefinitely.
According to those present, Prasad pushed for a motion to vote on the draft guidance, despite opposition from attendees who testified at the meeting. The other subcommittee members refused to vote, and finally the motion to table the draft guidance passed unanimously. A randomized controlled trial would be needed to demonstrate clinical utility of NGS tests, Prasad said at the meeting. (Experts say such trials would be neither feasible nor ethical.)
First-of-its-kind anti-NGS policy
Had it been enacted, the plan would have created an inferior standard of care for the poor in Oregon, depriving these patients of access to targeted therapies, mainstream oncologists and experts on disparities in cancer care said to The Cancer Letter.
Being the first-of-its-kind formal policy proposal by a government entity for denying coverage, the rationale for this recommendation could potentially have been used by other state Medicaid programs as well as by private insurers dredging for reasons to deny payment for NGS tests and treatments they may point to.
The draft guidance in question was previously approved by Prasad’s subcommittee. Of the five members on the subcommittee, Prasad appears to be the only oncologist.
Druker said OHSU will do everything it can to prevent the draft guidance from becoming policy.
Your voices were clearly heard and I applaud the committee for ensuring that our most vulnerable patients have access to this cutting edge technology.
Brian Druker
“At the Knight Cancer Institute, we are committed to doing everything we can to ensure that patients have access to life-saving diagnostics and therapeutics,” Druker said to The Cancer Letter before the public hearing. “NGS testing is increasingly allowing us to individualize therapy, and it is vitally important that our policymakers understand that the opportunities NGS offers today are considerably more advanced than they were even two to three years ago.
“The most recent evidence strongly supports the clinical utility of NGS. We are well positioned to inform policymakers about these advances, so that Oregonians have access to this cutting edge technology.”
Earlier this year, the Centers for Medicare & Medicaid Services issued a National Coverage Determination for diagnostic laboratory tests using NGS for patients with advanced cancer. This means that patients eligible for Medicare will be able to receive NGS tests. Foundation Medicine’s FoundationOne CDx receives full coverage under the NCD, and several other tests receive coverage from some local Medicare contractors (The Cancer Letter, March 23).
The clinical utility of NGS testing and its role in precision oncology was the subject of debates at the 2018 annual meetings of the American Association for Cancer Research and the American Society of Clinical Oncology.
At both debates, Prasad, the chair of the Oregon subcommittee, took the con side, arguing against NGS testing and precision oncology (The Cancer Letter, June 22, Sept. 7).
“I believe that in oncology, we cannot practice improvisational oncology. We cannot just merely have hunches, and let the average community doctor just prescribe drugs based on a Foundation Medicine report,” Prasad said at the AACR debate. “And yet, that’s precisely what is happening in this country day in and day out, we have rampant off-label studies being performed.
“My conclusions: The rhetoric has outpaced reality; there are true successes here, but few,” Prasad said. “Sequencing and drug should be paid for with research or commercial funds until proof of benefit. CMS, unfortunately, is not a research funder, they cannot be used that way. They have fiscal difficulties themselves, and they have to pay for services that have proven benefit.
“Is genome-informed cancer medicine generating patient benefit or just hype? I conclude that there is some benefit, but it is mostly hype.”
The draft guidance prepared for the Sept. 27 meeting of the Health Technology Assessment Subcommittee concludes: “Next generation sequencing tests of solid tumor tissue are not recommended for coverage (strong recommendation).”
The document is posted here.
The recommendation was unanimously approved for public comment by Prasad and three other subcommittee members on June 28, minutes show. Adam Obley, an assistant professor of medicine in the OHSU Division of General Internal Medicine, conducted the review of evidence for the Medicaid draft guidance.
As chair of the subcommittee, Prasad was not “actively involved in the development of an initial draft coverage guidance before it is presented to the subcommittee,” OHA officials said in a statement Sept. 26 to The Cancer Letter. “An OHA contractor (Center for Evidence-based Policy) writes the draft evidence summary portion of the draft coverage guidance. OHA/HERC staff write the draft coverage guidance recommendation based on the evidence summary. An initial draft of the coverage guidance goes to the subcommittee and is posted online in preparation for the public meeting.
“The chair has no role in writing the initial draft,” OHA officials said. “They see a copy of the draft recommendation during a leadership call to prepare for the first meeting where it will be discussed. No changes typically result from the leadership call. At the HTAS meetings, subcommittee members review the draft guidance, review public comment, and then deliberate about the recommendations. Changes to the draft guidance are made during these public subcommittee meetings.”
Jumping from academic debate to coverage
While the ASCO and AACR debates were academic, the Oregon proposal amounted to an attempt to translate arguments previously voiced by Prasad into policy, critics say. Prasad did not respond to an email from The Cancer Letter.
In the draft guidance, the subcommittee said that there is no direct evidence of clinical utility of NGS tests:
“Published evidence is insufficient at present to establish the balance of benefits and harms associated with next generation sequencing,” the subcommittee wrote. “The potential benefit of broad companion diagnostic testing has not been established by clinical utility studies. There is also potential harm related to the use of next generation sequencing in promoting the use of more costly targeted therapies when equally effective (or more effective) conventional chemotherapy might be available.
“The impact of next generation sequencing on clinical outcomes (cancer-related morbidity and mortality) or clinical decision making has not been established. A single randomized controlled trial showed that molecularly targeted therapies perform no better than treatments selected at the clinician’s discretion for previously treated patients with metastatic solid tumors. Resource allocation would be significant for next generation sequencing and the associated targeted chemotherapy agents.
“Although personalized (precision) cancer therapy is of significant interest currently, our recommendation for non-coverage of next generation sequencing tests is strong because there is no direct evidence of benefit, and the best available evidence does not yet establish survival advantage with targeted cancer therapies.”
Creating a disparity
Had the Oregon Health Authority approved the subcommittee’s recommendation, Medicaid beneficiaries in the state will be placed at a greater disadvantage vis-à-vis wealthier Oregonians, said John Stewart, associate director of clinical research at the University of Illinois Cancer Center, University of Illinois at Chicago.
“The unwillingness to provide patients with targeted therapies based upon their genetic profile, I think, is unconscionable,” Stewart said to The Cancer Letter. “It would create a therapeutic divide between patients with high socioeconomic status and patients with lower socioeconomic status.
“[The draft guidance] does not make sense. The logic to me behind that is, ‘It’s okay to be elderly and sick, but it’s not okay to be poor and sick.’ That’s how the draft guidance reads to me, because you won’t have access to state-of-the-art diagnostics. This is potentially an assault on the treatment of underrepresented populations for cancer.”

A conversation with Stewart, who reviewed the draft guidance for The Cancer Letter, appears here.
Prasad should have recused himself from deliberations on policy recommendations against coverage for NGS tests, said Richard Goldberg, director of the West Virginia University Cancer Institute and the Mary Babb Randolph Cancer Center.
“I would say that he is not an unbiased evaluator of this technology,” Goldberg said to The Cancer Letter. “I would’ve said, ‘He should have.’ Because, if nothing else, it’s raising the question of: Is he grandstanding? Or is he being an unbiased judge?
“If I were in his situation, I would’ve recused myself.”
Goldberg, who reviewed the draft guidance for The Cancer Letter, said the proposal would widen disparities in cancer treatment.
“My opinion is that patients should have equal access to technology that is becoming useful in improving outcomes, regardless of which insurer they are covered by,” Goldberg said. “There shouldn’t be disparities between private-paying insurers and government-paying insurers, and government insurers like Medicare vs. those that cover low-income individuals.
“I can tell you that every week, we’re doing NGS tests on Medicaid patients in West Virginia as well as on patients with every other kind of insurance.”
A conversation with Goldberg appears here.
Are RCTs feasible? Ethical?
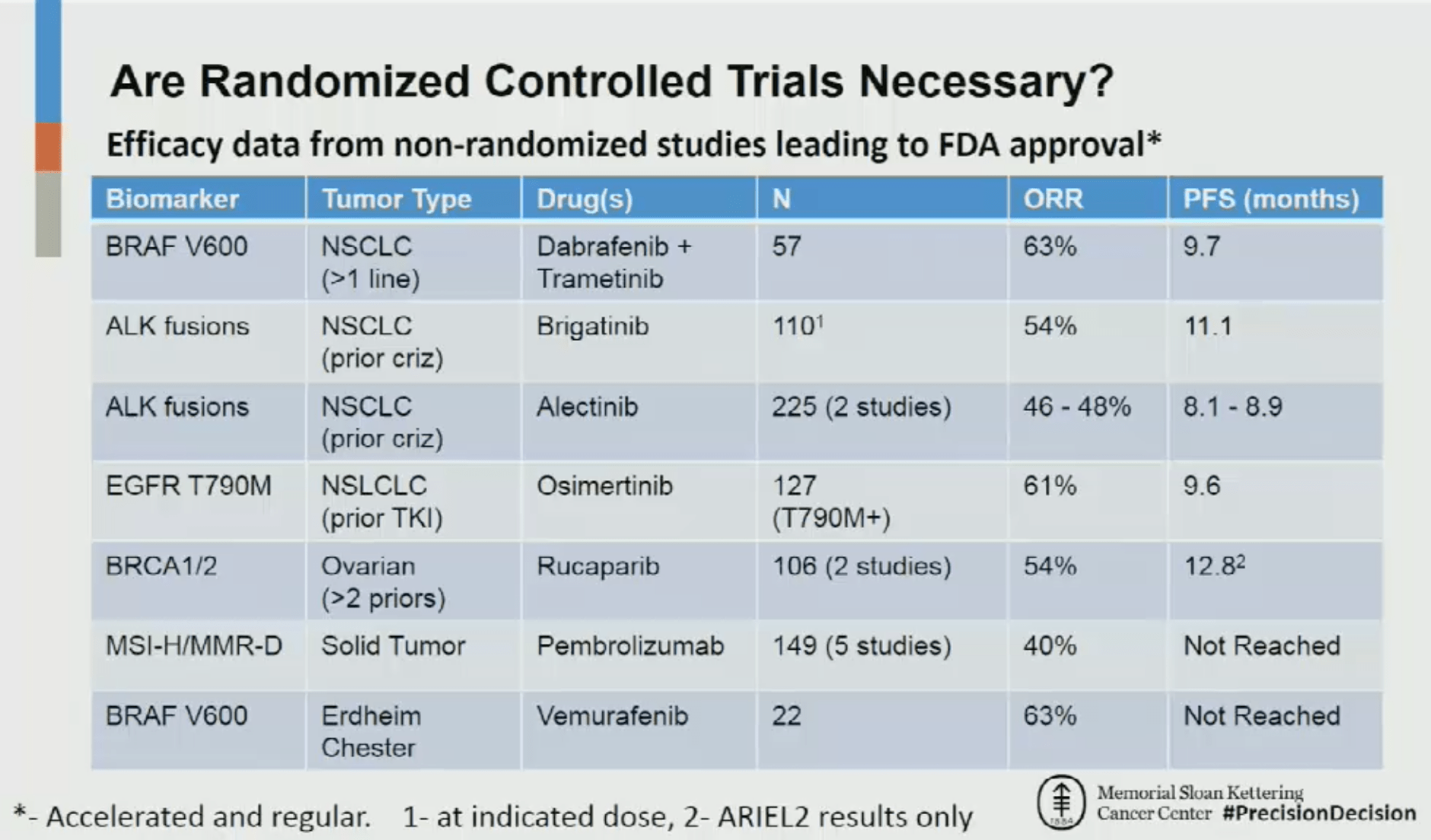
In a debate with Prasad at AACR’s annual meeting earlier this year, David Hyman, chief of the Early Drug Development Service at Memorial Sloan Kettering Cancer Center, said:
“What I did just for my own edification and knowledge, I went through the past seven FDA-approved—and this is inclusive of both regular approval and accelerated approval—indications based on a biomarker-selected population where the evidence was generated using single-arm, non-randomized, non-blinded data,” Hyman said at the time. “What you could see quite clearly is an overall response rate hovering in the 50+ percent range, with a median progression-free survival typically exceeding 9 months.
“When I look at this, I actually feel quite comfortable with prescribing based on this level of evidence,” Hyman said.“I might actually go one step further and say that it would be potentially unethical to randomize patients after evidence like this was generated.”
The AACR session is posted here.
The Oregon draft guidance was excessively restrictive to patient access and innovation in precision medicine, said Jeff Allen, president and CEO of Friends of Cancer Research.
“The analysis conducted mistakenly conflates evidence regarding clinical utility of a NGS test with effectiveness of a targeted therapy and fails to fully understand the value of NGS diagnostics in patient care,” Allen said a comment submitted to the subcommittee. “We urge HERC to revise its recommendation of non-coverage for NGS diagnostic tests to support use of this tremendous technology and promote innovation for improved patient care.”
Jeremy Warner, associate professor of medicine and biomedical informatics at Vanderbilt University, said that oncologists get NGS tests for patients with advanced solid malignancies to:
Determine prognosis,
Determine one or more treatments that might slow down the cancer,
Determine that certain treatments will not help slow down the cancer, and
Look for inherited (germline) variants that might have implications for unaffected relatives, in the future.
Warner, the ASCO 2018 Annual Meeting Education Committee track leader of the Health Services Research, Clinical Informatics, and Quality of Care track, debated Prasad at the ASCO annual meeting earlier this year.
“Regarding #1, it isn’t necessary because prognosis is already very poor in this setting,” Warner said to The Cancer Letter. “Regarding #4, most commercial NGS tests actually scrub these results out, so unless the test is specific for germline, it isn’t going to report heritable implications. However, it is quite possible that today’s variant of unknown significance (which is reported) might be tomorrow’s inherited mutation; see link.
“So that leaves #2 and #3. For #2, it is certainly the case that there are no FDA-approved tumor-agnostic targeted therapies; the one FDA-approved immunotherapy, pembrolizumab, has a trigger based on MSI status, which would probably (but not definitely) be known without having an NGS test. Otherwise, all of the current evidence from basket trials is non-randomized and to my knowledge none of the drugs tested to date are recommended in a tumor-agnostic manner by compendia or guidelines.
“However, we are all awaiting the likely approval of larotrectinib for TRK fusions (regardless of cancer type) and the results announced at ASCO for LOXO-292 for RET fusions are promising as well. If and when these drugs are approved, it makes a strong case for NGS testing as there is only so much tissue and ‘one-off’ testing is going to exhaust the biopsy quickly.
“For #3, it gets complicated. For some, knowing that they had all the most modern testing and there are ‘no available treatments’ left can be closure of sorts (there is always best supportive care and palliative care, let’s not forget). For others, this could be seen as ‘taking away hope’ and could be psychologically damaging.
“Bottom line: For these sorts of complex tests with a panoply of possible results, a thorough discussion with the patient prior to ordering the test is paramount. And, of course, making sure to close the loop with a follow-up with face-to-face discussions. Unfortunately, in our fee-for-service driven practices, neither of these happens quite as often as it should.”
As written, the Oregon HERC is directing these patients only to toxic chemotherapy, said Vincent Miller, chief medical officer of Foundation Medicine.
“What’s at issue is that all patients diagnosed with cancer—regardless of economic status—have access to these tests, so that the full expanse of systemic treatment options, including FDA-approved targeted agents and mechanism-based clinical trials, can be identified for potential use,” Miller said to The Cancer Letter.
NGS tests can help physicians identify patients who may benefit from treatment with targeted therapies for a variety of cancer types, FDA said in a statement to The Cancer Letter.
“When FDA reviews these tests to determine their safety and effectiveness, we are assessing their analytical and clinical validity,” FDA officials said. “FDA does not require that test developers provide clinical utility as part of our review. However, some submissions may contain this.
“For example, sponsors often leverage therapeutic product clinical studies to support clearance or approval of companion diagnostics. Such trials may demonstrate clinical utility when the use of the test identifies which patients may benefit from a particular therapeutic.”
The NCD fine points
CMS March 16 published the final NCD that pays for NGS in a broad range of cancers (The Cancer Letter, March 23, Feb. 3).
The decision document is posted here. To qualify for coverage under the NCD, laboratories must meet three conditions:
FDA approval or clearance as a companion in vitro diagnostic;
An FDA approved or cleared indication for use in that patient’s cancer; and
Results provided to the treating physician for management of the patient using a report template to specify treatment options.
Labs that don’t have FDA approval or clearance may seek coverage from Medicare Administrative Contractors. MACs are usually reticent to provide such coverage.
Foundation Medicine clearly has benefited the most from the NCD.
Last November, the company’s FoundationOne CDx test received an FDA approval, and—concurrently—CMS issued a provisional NCD defining the settings where Medicare would cover the test (The Cancer Letter, Dec. 1, 2017). It was FMI that requested the NCD.
It’s left up to MACs to decide whether NGS tests provided by cancer centers, such as MSK-IMPACT, should be covered.
The NCD covers Stage III and IV, metastatic, recurrent, relapsed, or refractory cancers. The NCD provides coverage across all solid tumors.
Repeat testing is covered using the same diagnostic laboratory test using NGS in the same patient only when a new primary diagnosis of cancer is made.
The first joint CMS-FDA approval was given to Cologuard, a stool DNA test sponsored by Exact Sciences Corp. (The Cancer Letter, March 28, 2014).
There are two other NGS tests approved by FDA:
The Oncomine test, sponsored by Thermo Fisher Scientific, for targeted therapies for non-small cell lung cancer, and
Praxis Extended RAS Panel, used to detect genetic mutations in RAS genes in tumor samples of patients with metastatic colorectal cancer. The test is sponsored by Illumina Inc.
Both tests are approved for specific genes and tumor types. Neither Oncomine nor Praxis has gone through parallel review by CMS.
Paul Goldberg contributed to this story.













