An NCI-sponsored trial showed that up to 70 percent of women with hormone receptor-positive, HER2-negative, axillary lymph node-negative breast cancer would not benefit from chemotherapy.
The trial—called TAILORx, or the Trial Assigning Individualized Options for Treatment (Rx)—was presented at a plenary session at the annual meeting of the American Society of Clinical Oncology June 3.
A paper stemming from the study was simultaneously published in the New England Journal of Medicine.
About half of breast cancer patients diagnosed worldwide each year have hormone-receptor positive, HER2-negative, node-negative disease. TAILORx found that for the vast majority of women with this form of breast cancer, adjuvant chemotherapy and hormone therapy is non-inferior to hormone therapy alone.


TAILORx was designed and led by the ECOG-ACRIN Cancer Research Group. The trial, which began in 2006, is the largest ever conducted in adjuvant therapy for breast cancer. It is also one of the first large-scale trials to examine a methodology for personalized cancer treatment.
Altogether, the phase III trial enrolled 10,273 women and randomized 6,711 of them. The trial was conducted at 1,182 sites in the United States, Australia, Canada, Ireland, New Zealand, and Peru.
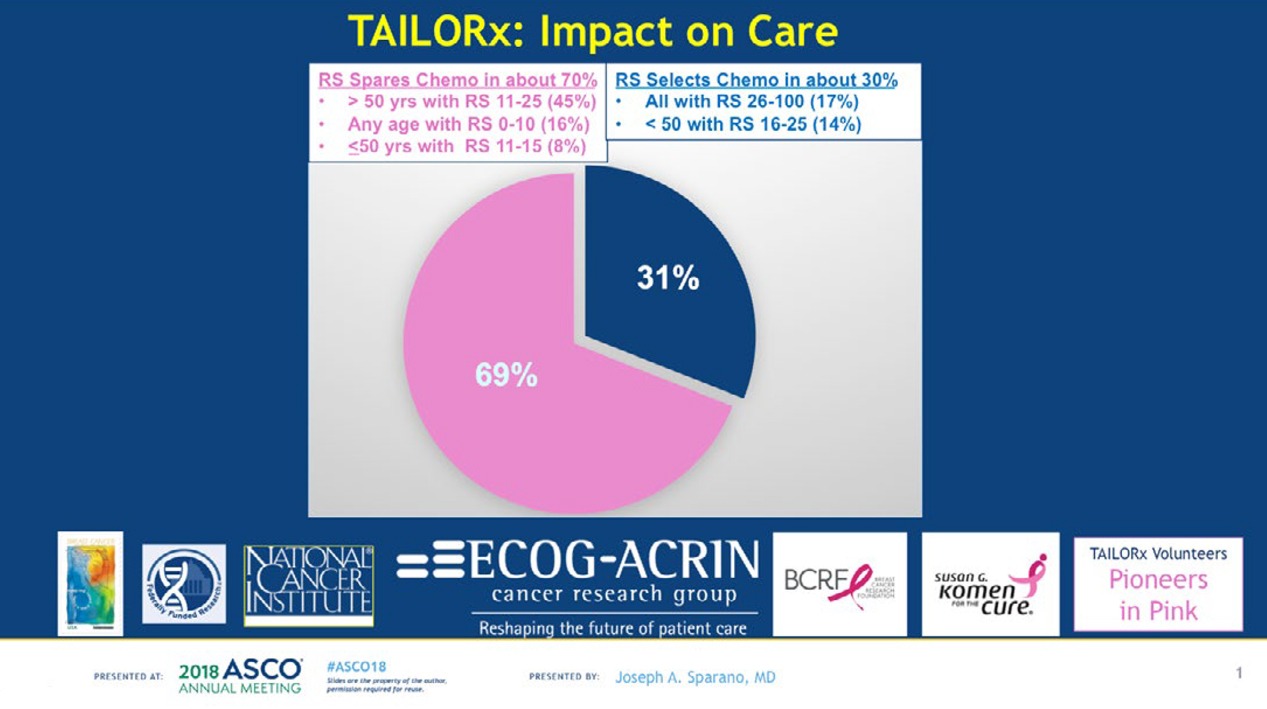
“In terms of the big picture and the impact on care, application of this test in clinical practice in this population will be estimated to spare chemotherapy in about 70 percent, and to select chemotherapy in about 30 percent, on average,” said Joseph Sparano, lead author of the NEJM paper, who presented the results at ASCO. Sparano is the associate director for clinical research at the Albert Einstein Cancer Center and Montefiore Health System and vice chair of the ECOG-ACRIN Cancer Research Group.
NCI doesn’t regularly tabulate the cost of individual trials, but a back-of-the-envelope calculation by Jeffrey Abrams, associate director of NCI’s Cancer Therapy Evaluation Program, suggests that over 12 years, the institute spent $35 million to $40 million on the trial. An additional $5 million came from the sale of the Breast Cancer Research Stamp.
“It speaks to the fact that we really do have a national network that can conduct studies like this,” Abrams said to The Cancer Letter. “This is the type of study that a pharma company would not do because it’s not intended to bring a new treatment into medicine; it’s really to decide how best to treat patients, and actually remove some treatments from patients.
“It shows that the government still has a role to play for this kind of trial.”


A conversation with Abrams appears on page here.
“I think [TAILORx] is important for several reasons,” Harold Burstein, associate professor at Harvard Medical School and staff physician at Brigham and Women’s Hospital, said at a press conference at ASCO. “First, the original data for use of the Oncotype DX recurrence score were based on chemotherapy regimens that were 25 years old now. So, the question has been, if we use modern chemotherapy, would the results be different?
“Secondly, we’ve improved our endocrine treatments, our anti-estrogen approaches, which also may have affected these results.
“Third, it’s important to have prospective validation of the data, and finally, there was a gray zone, an intermediate zone, where it was unclear what the magnitude of benefit for chemotherapy might be, and this created practical decision-making challenges for patients and for clinicians.


“For those of you who have ever been a clinician in a consultation room, or a patient in a consultation room, you know there is a huge difference between saying, ‘Well, you might benefit a little bit,’ and saying, ‘There is no benefit for you. ‘And with the data provided here, from this massive NCI-sponsored trial, show that the vast majority of women who have this test performed on their tumor can be told that they don’t need chemotherapy, and that can be said with tremendous confidence and reassurance.’
Experts’ commentary about TAILORx appears below.
In 2000, an NIH consensus conference recommended wider use of adjuvant chemotherapy for women with localized breast cancer. At the time, it was clear that a minority of women stood to benefit from adjuvant chemotherapy, but no technology existed to identify the women who stood to benefit.
Nearly two decades later, the hottest controversies at that consensus conference now look like a side issue—the role of paclitaxel in adjuvant therapy. (The Cancer Letter, Nov. 10, 2000).
TAILORx used a molecular test, the Oncotype DX Breast Recurrence Score, to assess the expression of 21 genes associated with breast cancer recurrence.
With these results, the use of Oncotype DX, which costs around $4,000, becomes even more hardwired into treatment decisions in breast cancer. Indeed, when the stock market opened on Monday, June 4, the price of a share of Genomic Health jumped from $39.7 to $43.9. It reached the high of $51 on June 6, and is now hovering around $49.
Oncotype DX doesn’t have FDA clearance, while a competing product, Mammaprint, has FDA 510(k) clearance. The Genomic Health central laboratory has the US Clinical Laboratory approval to offer Oncotype DX as a homebrew.
In TAILORx, researchers used the Oncotype DX score to assign women with early-stage, HR-positive, HER2-negative, axillary lymph node-negative breast cancer to adjuvant treatment.
Only women with Oncotype DX results of 11 to 25 were randomized to receive endocrine therapy with or without chemotherapy.
Women with the scores of 0 to 10 were assigned to endocrine therapy alone, based on the prior results from the NSABP B-20 study, which showed no benefit of chemo in this low-risk group.
Women with the score of 26 to 100 were assigned to chemotherapy and endocrine therapy, also based on NSABP B-20 study, which showed an absolute benefit of chemotherapy greater than 20 percent.
The primary endpoint was disease-free survival, based on recurrence of cancer in the breast, regional lymph nodes or distant organs, a second primary cancer in the opposite breast or another organ, or death from any cause.
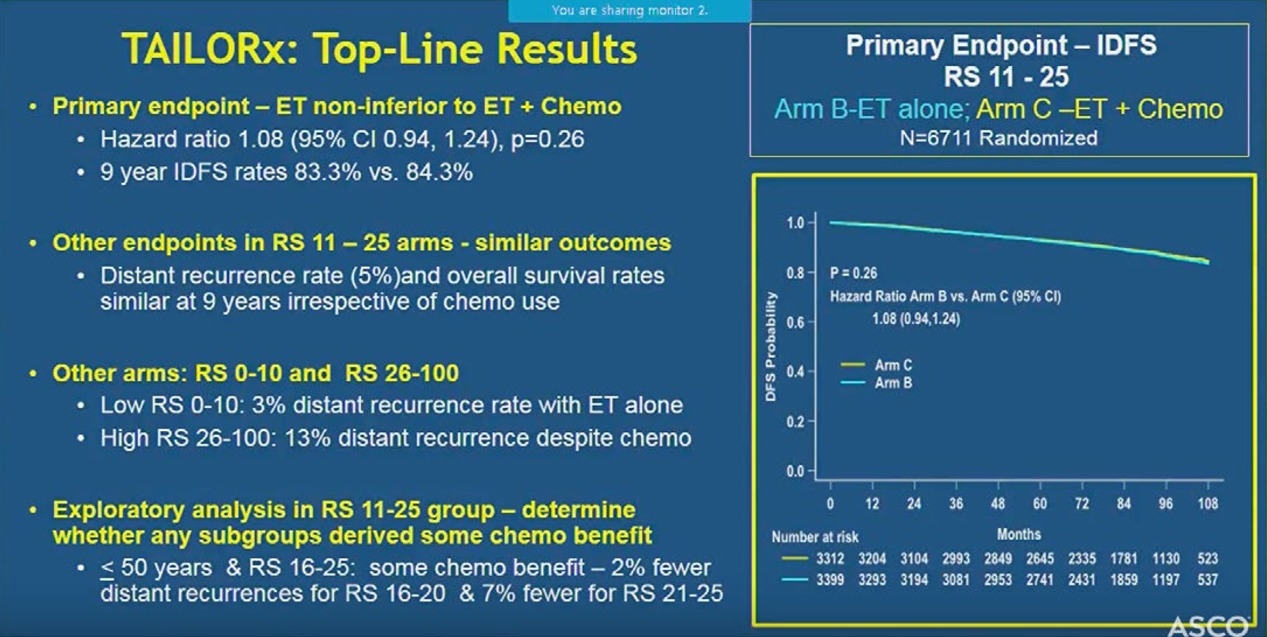
At a median follow-up of 7.5 years, the study met its primary pre-specified endpoint indicating that hormone therapy alone was not less effective than chemotherapy plus hormone therapy in women with a Breast Recurrence Score of 11-25.
Nine-year rates were similar in the two treatment arms for disease-free survival (83.3% vs. 84.3%), distant recurrence (94.5% vs. 95.0%), and overall survival (93.9% vs. 93.8%), indicating no benefit from adding chemotherapy to hormone therapy.
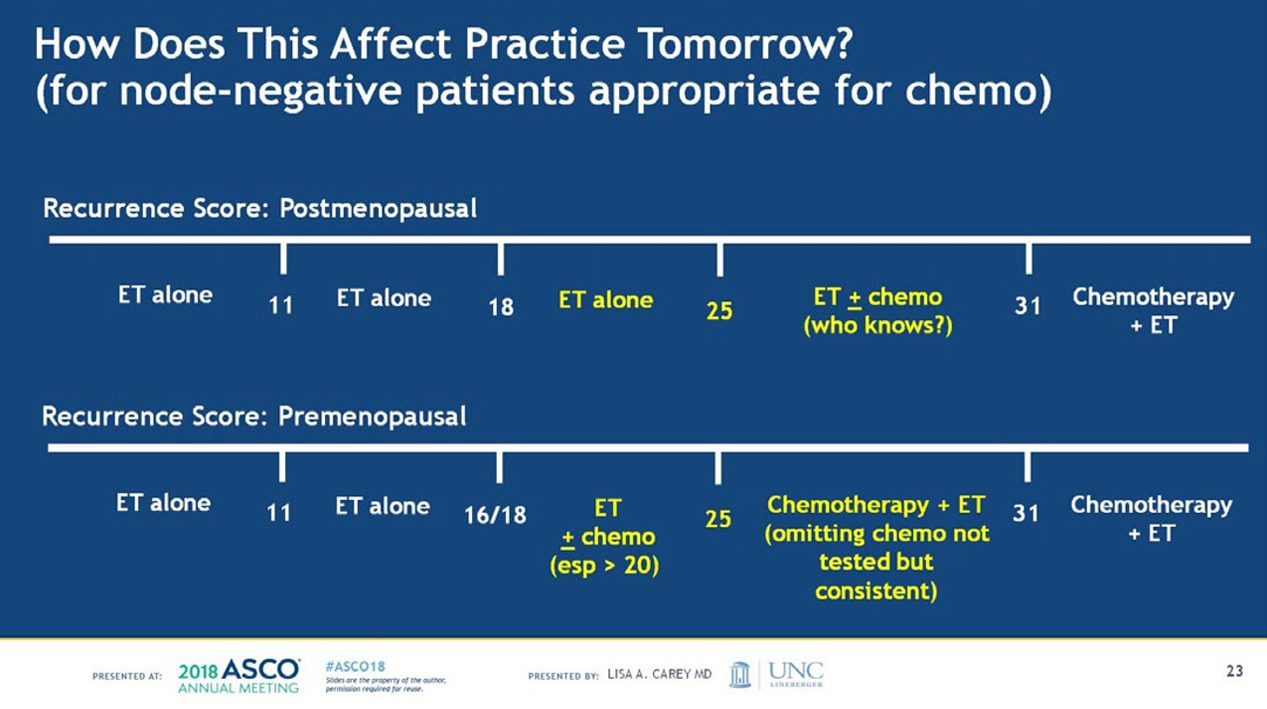


In a post-hoc analysis, the researchers identified the group that seemed to have some benefit from chemotherapy—women 50 years or younger who had a Breast Recurrence Score of 16-25.
“A very important finding was that in an exploratory analysis in a randomized group, which we conducted to really nail down and determine that there is no subgroup that derived some benefit from chemotherapy, we found an interaction between age and recurrence score,” Sparano said at a press conference at ASCO. “Younger women—50 or younger—who had the recurrence score of 16 to 25 had some chemo benefit, and there were 2 percent fewer recurrences for the recurrence score of 16 to 20, and 6 to 7 percent for those with a recurrence score of 21 to 25.
“And this is information that could drive some women who have the recurrence score in this range to accept chemotherapy.”
The researchers found that women with a recurrence score of 10 or less had very low recurrence rates with hormone therapy alone, irrespective of age or other clinical factors. In addition, those with a recurrence score of 26 or higher had a distant recurrence rate of 13% despite chemotherapy and hormone therapy, indicating the need to develop more effective therapies for this group.
According to the authors, the findings suggest that chemotherapy may be spared in:
All women older than 50 years with hormone-receptor positive, HER2-negative, node-negative breast cancer and a Recurrence Score of 0 to 25 (about 85% of women with breast cancer in this age group)
All women 50 years or younger with hormone-receptor positive, HER2-negative, node-negative breast cancer and a Recurrence Score of 0 to 15 (about 40% of women with breast cancer in this age group).
The researchers also found that women with a score of 0–10 had very low recurrence rates with hormone therapy alone at nine years (3 percent).
This confirms similar findings from earlier studies. In addition, they found that women with a score of 26–100 had a distant recurrence rate of 13 percent despite receiving both chemotherapy and hormone therapy. This finding points to the need to develop more effective therapies for women at high risk of recurrence.
“This is not so much about de-escalation, which is a phrase that many in the media had picked up on,” Burstein said at a press conference at ASCO. “The goal of this study is not just to use less treatment. The goal is to tailor treatment. They chose the title very aptly, with the idea that some women are going to need more of one kind of therapy and less of another and others are going to get a different treatment, based on the biology of their tumor.


“Even in the highest risk group of patients here, the ones that got chemotherapy and hormone therapy because their Oncotype scores were in the high range, the 10-year disease-free survival was 87 percent. We have made extraordinary progress in the way we are managing breast cancer. In the low-risk group, the recurrence rate is less than 5 percent.
“Women with breast cancer who are getting modern therapy are doing extraordinarily well, and this test shows us how to tailor that management so they get exactly the right amount of treatment—not too much and not too little.”
Lisa Carey, the discussant at the plenary session focused in part on the subgroup analysis that pointed to a potential benefit for a narrow group of patients.
“A couple things that we have to note: the first is this is an exploratory analysis. It’s also an unplanned analysis,” said Carey, the Richardson and Marilyn Jacobs Prior Distinguished Professor in breast cancer research and the chief of the division of hematology oncology at the University of North Carolina at Chapel Hill. “So, it’s an exploratory unplanned analysis of a very large study. It is, however, biologically plausible. We do know that, for example, chemotherapy has a substantial impact on ovarian function.
“And it is entirely possible that this is an impact of chemotherapy on ovarian function. We now know that ovarian suppression does improve outcomes in this setting. And so, it’s possible that that’s what we’re measuring here. It’s also possible there are other features of premenopausal disease that we have not taken into account.”
What experts say:


Chief medical officer, American Society of Clinical Oncology
We talk a lot at these meetings about diagnostic tests and whether they are well validated or not, and the series of studies over many years now, culminating in TAILORx, all performed using the Oncotype DX test is a paradigm for how diagnostic test development should be done, going from the retrospective analyses of completed clinical trials all the way to a large prospective clinical trial that shows such a definitive and important role for this particular test.
But I am not talking so much about the test itself as about the process, which I think is important to keep in mind.
TAILORx is a triumph for the publicly-funded cancer clinical trials system in the U.S., and once again demonstrates the crucial role played by the National Clinical Trials Network in answering clinically important questions that can change practice overnight.
The study also demonstrates the importance of public-private partnerships in advancing research and ensuring access to care. In my mind, the clinical development of Oncotype DX over the past two decades and culminating in TAILORx, provides a prime example of how molecular diagnostic tests should be developed and validated to provide high level evidence of their clinical utility.
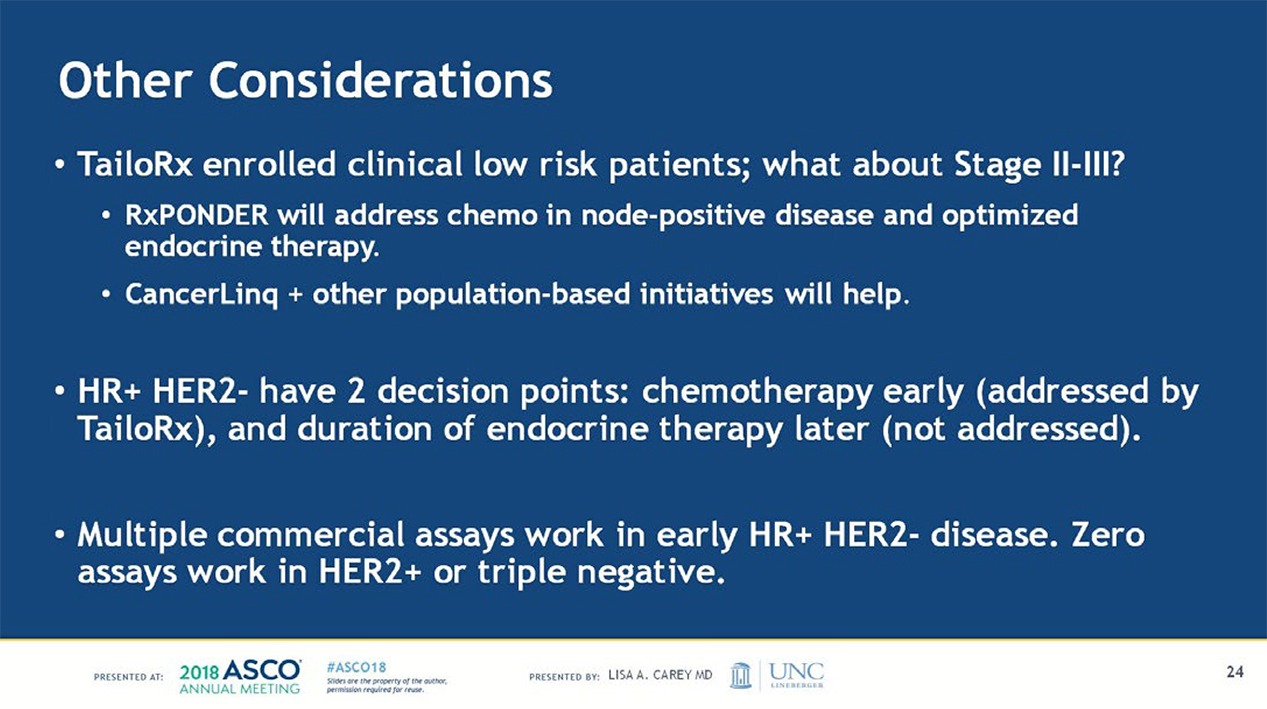
As is always the case with research, there are still questions to be answered, and an important one, in my mind, is whether the 21 gene recurrence score can be used to identify a subset of women with hormone receptor positive, HER2 negative, node positive breast cancer who can also safely avoid adjuvant chemotherapy. This might require another study on the scale of TAILORx, although since the event rates are likely to be higher in this higher risk population, I would hope that such a study could be completed with fewer patients and shorter follow-up.
This study was initiated by ECOG, and I would be remiss if I didn’t acknowledge the key role of my good friend and colleague, Dr. Bob Comis, who was the group chair of ECOG when the study was conceived. He was the driving force in bringing it to fruition, and who, sadly, passed away last year. This is such an important part of Bob’s legacy.


Director, Womens Cancers Program; vice chair, medical oncology; professor, Division of Medical Oncology & Experimental Therapeutics; associate director for education and training; Baum Family Professor of Women’s Cancers; City of Hope Comprehensive Cancer Center
It is comforting that chemotherapy did not benefit postmenopausal women with recurrence scores of 11-25. How then can we explain the small benefit of chemotherapy to premenopausal women in the subset analysis? The benefit from chemotherapy in this group is smaller than we generally see from chemotherapy and raises the suspicion that the “benefit” observed with the addition of chemotherapy may be due to ovarian suppression by the chemotherapy. It may be that premenopausal women with higher recurrence scores should receive ovarian suppression in addition to tamoxifen rather than tamoxifen with chemotherapy.


President, The National Breast Cancer Coalition
I very much hope this is sufficient evidence for the medical oncology community to walk away from chemotherapy for the vast majority of women with ER-positive early breast cancer.
The data have shown for many years that toxic chemo in this population is not effective, at all ages, and particularly in women over 50. But all these women face the significant harms that come along with chemo.
I often wondered why these women continued to be subjected to this treatment and what would convince doctors to stop.
We need criteria to take away less effective and harmful drugs when the data warrant and to make certain we add these toxicities only when the highest level of evidence tells us it will save lives. That would be a paradigm shift.
I remember us, NBCC, being involved from the very beginning, giving input on the protocol, doing outreach for the trial. Carolina Hinestrosa was part of the steering committee and Musa Mayer was on the DSMB. I recall many in the oncology community saying that women would never enroll in a trial like this, and forgo chemo.
At NBCC, we knew women had the courage and vision to join the trial. We never doubted that.


Chief clinical strategy officer, chief, of the Division of Women’s Cancers, senior vice president for medical affairs, chief of the Division of Breast Oncology Center, Susan F. Smith Center for Women’s Cancers, Thompson Chair in Breast Cancer Research, institute physician at Dana-Farber Cancer Institute and professor of medicine at Harvard Medical School
Overall, the study will not have a dramatic impact on our practice at Dana-Farber, because we have tended to give less chemotherapy to patients with node-negative ER+ breast cancer.
We did not expect to see a significant overall benefit of chemotherapy in the trial. But it will make us even more comfortable omitting chemotherapy in virtually all women who are over 50 and have a node negative cancer under 5 cm with an Oncotype of 25 or less.
In women 50 and under with an Oncotype of 21-25, we will have long conversations and weigh the pros and cons of chemotherapy.
Almost certainly, some of the benefits of chemotherapy in women 50 and younger arise from the impact of chemotherapy on ovarian function, and we have far less toxic ways of suppressing ovarian function.
With this in mind, I doubt that we will recommend chemotherapy to all patients under 50 with RS of 21-25, but we will discuss it. There is no question that the study is good news for women with node-negative, ER+ breast cancer.


Chief medical and scientific officer, American Cancer Society
“The TAILORx result is far more than a success in that it will save thousands of women from unnecessary treatment.
It is an example of how cancer medicine is evolving. For more than 150 years, cancer was diagnosed with a biopsy and a microscope. Today, the diagnosis of breast cancer involves a biopsy, a microscope and genomic testing.
We have moved from a mid-19th century definition of cancer to a 21st century definition of cancer.
Breast cancer was once one disease. Today, it is a number of diseases, each defined by genomic analysis. Each with a different prognosis and deserving treatment of varying aggressiveness.
With the discovery of the estrogen receptor and HER2/neu, breast cancer was one of the first cancers to so clearly become multiple diseases.
Cancer, in general, is trending toward more personalized medicine and many organ-specific cancers are moving towards being considered a series of different cancers.
For example, the numerous genetic mutations associated with non-small cell lung cancer almost make it a series of orphan diseases.
Progress comes with a cost. This will complicate efforts to develop some treatments and increase the complexity of clinical trials.


Vice president, clinical research, professor of medicine, MD Anderson Cancer Center
The results of this study were surprising; as addition of chemotherapy resulted in no improvement in disease free survival in this intermediate risk subgroup.
However, it is consistent with what we understand about endocrine therapy today. In earlier retrospective studies, the endocrine therapy consisted of tamoxifen therapy as an adjuvant.
Over the years, adjuvant endocrine therapy has evolved from tamoxifen to aromatase inhibitors, aromatase inhibitors are superior than tamoxifen in the adjuvant setting.
Thus, it is not surprising that any small benefit of chemotherapy in this intermediate risk group would not be detected with improved adjuvant endocrine therapy. Adjuvant chemotherapy in a subgroup with no gains in disease-free survival will only add side effects, like treatment-related leukemias and/or rare fatal infectious complications.
The physicians have the responsibility to inform patients about the results of this well designed study. As a number of women may still want to accept additional risks to reduce their risk of recurrence and want to take systemic chemotherapy.


Chief visionary, Dr. Susan Love Research Foundation
For too long in breast cancer the model has one of addition!
The TAILORx study is a powerful example that more is not better in treating breast cancer. This is important in terms of the cost of care both financially as well as in long term collateral damage from treatment.


Stuart B. Padnos Professor of Breast Cancer Research, University of Michigan Rogel Cancer Center
Great to see this come to completion. We’ve been working on this trial since 2003. Joe Sparano has done a great job running it, and his presentation was, in my opinion, exciting, but very thoughtful. Kudos to him.
One “side” conclusion, raised by Lisa Carey, is that patients diagnosed in the 2000’s with ER positive breast cancer are doing better than those diagnosed (and put on trials) in the 1970-’80s, from which we drew our assumptions to power TAILORx.
Breast cancer research and treatment has been special in two ways, in my opinion:
The biology (ER, HER2) has made it amenable to targeted, and effective, therapies for decades, and furthermore it has just been more chemosensitive than the other common solid tumors
The approach taken by our surgical forefathers–we all owe Drs. Fisher, Bonadonna, Danforth, Crile, and others, coupled with their radiation oncology partners, for courageously challenging Halstedian dogma and “de-escalating” surgical approaches. Almost unheard of in the other diseases.
We (medical oncologists) justifiably spent the first 40 years of the field (1960-2000 or so) “escalating”–because we had to.
The reduction in breast cancer mortality over the last 30 years (by as much as 1/3-1/2) is a gratifying result. But, now we take a page from their book and see de-escalation with no detriment in this reduction in mortality–terrific.
Taken together, the advances in biologically-based therapies and now personalized delivery of chemotherapy will continue to improve both the length, and quality, of our patients’ lives.
We now look forward to:
Results from the RxPonder trial – can we further reduce therapy that is either not needed or won’t work? Can we expand the percentage of patients whom we will no longer over-treat while simultaneously getting the “right drugs to the right patients at the right time, dose and schedule?”
New therapies that continue to take advantage of the cancer biology – PARP inhibitors, etc.


Chief scientific officer and chief medical officer, Genomic Health
TAILORx, as well as the completed NSABP B-20 Oncotype DX study, are unparalleled in their design to define who does, and who does not, benefit from chemotherapy. The long-term TAILORx results provide the highest level of evidence for Oncotype DX, allowing physicians to now tell every patient, with precision, what their magnitude of chemotherapy benefit will be.













