NCI awarded eight new, competing and renewed grants as part of its funding for its Specialized Programs of Research Excellence. The grantees will receive $2,185,000 per year for five years.
THE DEATH OF CANCER After Fifty Years on the Front Lines of Medicine, a Pioneering Oncologist Reveals Why the War on Cancer Is Winnable–and How We Can Get There. By Vincent T. DeVita, Jr. and Elizabeth DeVita-Raeburn; Illustrated. 336 pp. Sarah Crichton Books, Farrar, Strauss & Giroux. $28.00“The Emperor of All Maladies” was a history of oncology, and a good one. “The Death of Cancer” is a memoir of one of the greats of medical oncology. It is a history from someone who was there, making history.
Vincent T. DeVita Jr. has seen the cancer field as a confident young doc eager to challenge the system, as a general in the War on Cancer, as an academic oncologist and, most recently, as a patient.
A Boston judge ruled Nov. 3 that Brigham & Women's Hospital had violated the First Amendment rights of a couple who led an aggressive national campaign to stop power morcellation, a surgical procedure routinely used by gynecologists.
Hospitals serving large populations of low-income patients stand to lose up to seven figures a year in drug discounts if proposed regulatory changes to the 340B program are enacted, the program's supporters say.
Congress passed a two-year budget deal that would raise government spending as well as the debt ceiling.
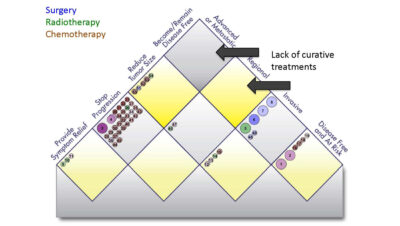
Lilly Oncology has launched a novel value assessment tool that aggregates 40 years of oncology data to measure progress and identify unmet needs in cancer treatments.
Eli Lilly & Co. didn't ask Dan Goldstein, an oncologist at the Winship Cancer Institute at Emory University, to price their drugs, but he volunteered his services anyway.
The American Cancer Society published a breast cancer screening guideline that steers toward the middle course in deciding when mammography screening should start and how often it should be performed.
The Cancer Letter invited Richard Wender, chief cancer control officer of the American Cancer Society, to describe the rationale for the society’s new guideline for breast cancer screening.