This story is part of The Cancer Letter’s ongoing coverage of COVID-19’s impact on oncology. A full list of our coverage, as well as the latest meeting cancellations, is available here.
Since the dawn of man: when a novel virus is introduced to the human species, the world is changed forever. Despite all of our advances—we can share information around the globe in seconds and we can fly to the moon—a never-before-seen virus can stop us all in our tracks and steal people’s lives too soon.
At The University of Texas MD Anderson Cancer Center, we began closely monitoring and tracking the activity of the SARS-CoV-2 virus as the virus and the news started to spread in early January.
It quickly became clear that the potential for a global pandemic was real. We knew we needed to be prepared, and we started informing our employees about the virus, ordering PPE, and formulating a crisis management plan, to have it ready by late January. It was helpful to have an enterprise risk management team and a program in place that gave us a strong foundation in dynamic risk calibration, modeling and management.
MD Anderson has one of the largest and densest concentrations of immunocompromised patients in the world. We consider it our responsibility to protect the health and safety of all of our patients at all times, including during a global pandemic. As we started seeing data coming from impacted countries that showed that cancer patients who contracted the SARS-CoV-2 virus are at increased risk for hospitalization and death from the infection, we knew the months ahead would require swift, significant action to ensure we could fulfill our commitment to patients.
To guide our path forward during what we knew would become a global pandemic, we established three goals to inform all decisions: protect our uniquely vulnerable patient population, ensure the health of our workforce, and minimize disease impact on our local community.
It is important to highlight that from the start of this pandemic, we made thousands of decisions—all with a focus on protecting our patients and employees. Many were good, some were great, and others we would not do again, but—most importantly—we have learned so much along the way, and our organization has become stronger as a result.
As a leader, I also have been tested, I have grown, and I have learned many lessons over recent months. Multi-channel communication has been fundamental to our efforts. We have prioritized daily, fact-based, transparent communication with our workforce, sharing guidance on the decisions being made and resources available.
Some of our communications tools have included: daily COVID-19 briefings each morning, often with as many as 600 employee leaders joining the 30-minute WebEx for updates; daily emails from me, with further updates; nearly 30 short videos from me distributed regularly; a comprehensive employee COVID-19 app; and virtual livestreams and town halls that have brought as many as 16,000 employees together for the live, 60-90-minute meetings. These efforts were vital, because while communication is an important part of leadership at all times, communication is essential during a crisis.
Collaboration also has been key to our response. We have partnered with other Texas Medical Center (TMC) health systems to closely work together on issues including communications and supply chain, to share information, and to help inform the public—via media and social media—about the realities of this virus.
We have worked with elected city and state officials to keep them informed—especially when difficult decisions needed to be made. We also remain connected with national groups and our peer institutions across the nation to share data and to learn from one another.
This has been a time for great partnership as we tackle this global challenge together.


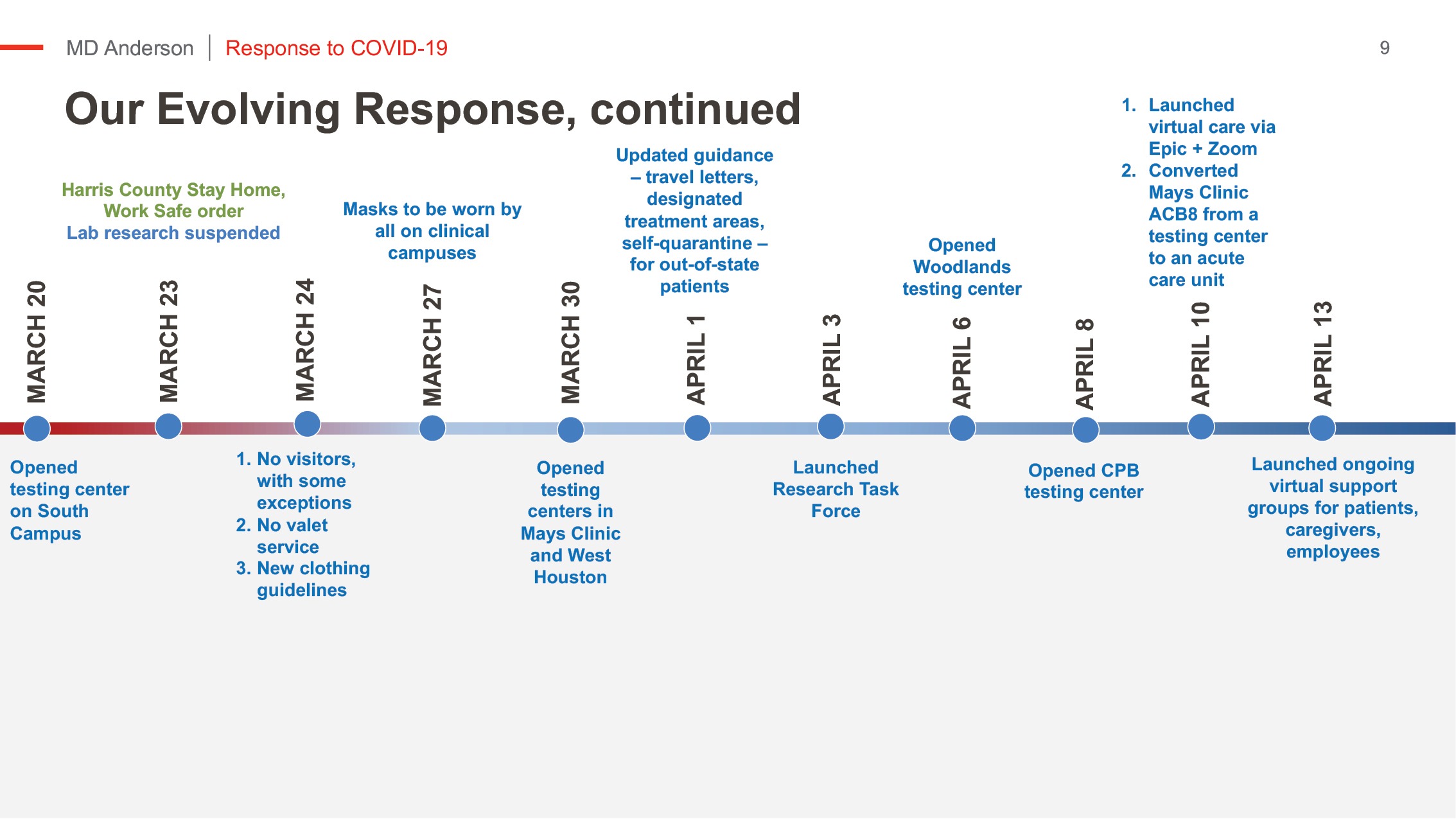



MD Anderson’s clinical response
Starting in early March, we moved to scale down our enterprise in response to federal and state guidelines.
The speed at which some actions had to be taken felt as though a series of light switches were turned off. It was not easy or without pain, but the dedication and commitment of MD Anderson’s faculty and staff was extraordinary.
Our effort has involved a focus on short-term immediate crisis response along with assessing long-term business continuity needs. With these time frames in mind, we created a COVID-19 Core Leadership Team to act on immediate priorities and an Enterprise Resiliency Executive Oversight Committee to consider medium-to-long-term risks and opportunities.
Decisions made in those forums are reviewed by the President’s Advisory Council, allowing leaders from all segments of our institution to provide perspectives and to help support sense-making in their work areas. All of these groups provide guidance and insight to inform my decision-making process.
It is important to highlight that these committees include division heads, department chairs, faculty senate members, executive leaders, and faculty and employee representatives, providing diversity of opinions from across our institution. Meetings have been frequent, discussions have been thorough and decisions are made with great thought, always keeping our three goals in mind.
Much of the outstanding committee work has been led by Rosanna Morris, chief operating officer, and Welela Tereffe, M.D., chief medical officer. Additionally, we have learned that Dr. Tereffe is an expert host and moderator of our frequent leadership and institution-wide briefings.




Since January, thousands of decisions have been made. A sampling of those decisions includes:
As early as in February, we started cancelling business travel and, as virus activity progressed, we asked staff to log personal travel in a central registry. At one point, all personal travel outside the state of Texas required a mandatory 14-day quarantine upon return.
We created a perimeter around our clinical care areas to limit access points, and we coordinated symptom screening, temperature checks and masking for all.
We reduced onsite employees at our TMC campus from a typical day of 16,500 people to, on average, only 6,500 people.
We expanded PPE protocols to ensure everyone was safe and protected. PPE supply chain and donations allowed us to go above CDC standards throughout our experience thus far.
We implemented a zero-visitor policy with few exceptions, and we required social distancing measures on campus.
We launched our virtual care platform to continue serving patients unable to travel, and we tapped into MD Anderson’s national network of hospitals to offer some patients care close to home.
We knew the importance of testing and moved early to stand up our own capabilities. We offer these services broadly to MD Anderson patients and employees at locations across Houston. We have the capability to conduct 1,200 tests per day, providing results within 36 hours; most are available within 24 hours.


To provide added assistance to those in our community and across Texas, we also conduct testing for county hospitals in the Harris Health System and for the Texas Department of Emergency Management.
As of June 9, we have tested nearly 8,000 MD Anderson patients. Of those, 103 patients have tested positive for COVID-19 and 38 have been treated in our inpatient hospital. Sadly, four patients have died, making our mortality rate significantly lower than published data from other countries.
More than 2,200 employees have been tested; 126 have been COVID+ and most already have returned to work. Among more than 22,000 employees, about .5% of our workforce have been infected. We attribute these low rates to our employees’ shared commitment to proper use of PPE, social distancing, and hand hygiene—at work and at home.
We also expanded our offering to include asymptomatic testing for any employee who requests it. More than 600 employees have undergone asymptomatic testing and only four have tested positive.
We fully recognize that this crisis has both physical and emotional impact on our employees. Consequently, we have increased our focus on ensuring the safety, the security and the physical and emotional well-being of our workforce.
To understand employees’ concerns at scale and over time, we conduct pulse surveys on a frequent basis. With regard to employee worry and anxiety, we want our workforce to be able to tangibly grasp that we understand how stressful this period in time is for them and for their families.
Our second employee pulse survey garnered responses from 71% of our employees from all areas of the institution. More than 88% of participants believe their health and well-being is a top priority for MD Anderson; 86% feel supported by their managers in making decisions about health and well-being; and nearly 95% believe MD Anderson is doing what is necessary to support our patients during the pandemic.
The results of these surveys help inform our continued efforts to support our employees’ needs and to enable us to target resources and attention to specific units that may need more support.


Importantly, we also offer counseling and access to financial support through our Caring Fund, an employee assistance fund that allocates grants to co-workers who are experiencing undue hardships as a result of the virus.
Anyone can contribute to the fund via online donations, and MD Anderson employees can elect for payroll deduction. We have funded disaster leave for employees unable to work their full hours to maintain their health insurance and full benefits.
We also created an opportunity for employees to donate vacation and extended illness benefit hours for others to use, and the response has been unbelievable: 60,000+ hours have been donated, to date.
Research response was difficult, but necessary
At the end of March, as daily case volumes in the Houston region jumped, and trajectories showed Houston tracking with and at times ahead of Wuhan and Lombardy, basic and clinical research efforts were modified or suspended in order to protect our patients and employees.
With the pandemic accelerating, a difficult decision was made by our Chief Scientific Officer Dr. Giulio Draetta to require researchers to safely decommission ongoing experiments, store reagents in the appropriate manner, and power down instruments for an unknown length of time. Valuable research tools, including animal models, cell lines and reagents, were preserved as much as possible to enable continued research when laboratory activities resumed.
Impressively, during an extremely difficult time for our researchers, they collected critical reagents and PPE supplies found in their laboratories and donated them to support our clinical teams. We take great pride in these acts of collaboration and commitment to the greater good of our institution and to our patients.
Where possible, researchers have worked remotely, and outstanding science has been generated during this time, including work focused on COVID-19 and efforts focused on grant-writing and fostering future collaborations. In response to the numerous ideas and proposals put forth for meaningful research focused on the virus and associated disease, we established the COVID-19 Research Task Force.
Through the guidance of several focused workgroups, the task force is responsible for prioritizing and advancing COVID-19 research projects. Already, two MD Anderson research projects were chosen by the National Cancer Institute to advance for full proposals. We also are collecting plasma donations from those who have recovered from COVID-19, and we are participating in a national initiative to provide that plasma to seriously ill COVID-19 patients.
We also established our Data-Driven Determinants for COVID-19 Oncology Discovery Effort (D3CODE), which created a cross-functional, institution-wide data science initiative linked to the pandemic. Under IRB approval, we activated a protocol to facilitate data-focused research related to COVID.
This eliminated the need to submit individual protocols solely for the purpose of aggregating and analyzing this data. The goal of this initiative is to provide investigators with a common, curated and readily available source of aggregated COVID+ patient data to empower the rapid prosecution of research questions.
Modifications also had to be made to clinical trials to ensure patients remain safe and receive the care they need, while reducing in-person interactions to only those that are essential for the provision of safe medical care.
Throughout the pandemic, many of our clinical trials continued using remote monitoring and virtual care. Clinical trials that could not continue using remote methods of interaction were temporarily suspended.


Education continues, with modifications, during our response
Our education mission has continued throughout our response to COVID-19. Early on, our educators worked to develop training for our patient care teams on proper PPE use and patient care best practices to safely care for COVID+ patients.
Our fellows and residents have continued to be integral parts of our care teams, and those who have expressed interest in caring for our COVID+ patients have been invited to participate in this learning opportunity, when possible.
Our School of Health Professions (SHP) has continued courses online and is hosting virtual open houses for prospective students. Many of our students have supported important efforts during the pandemic. A great example is that current SHP Molecular Genetic Technology students assisted with our routine diagnostic testing while our Molecular Diagnostic Lab employees focused on developing and processing COVID-19 tests.
At our Graduate School of Biomedical Sciences, students have been successfully defending their theses over web conferences in preparation for graduation. Many of our summer research programs also are creating virtual experiences to engage high school, college, graduate students and postdoctoral fellows in science and research lectures, career advice and more.
Our commitment to educating future cancer health care providers remains strong, and we are working to safely support their learning during this unprecedented event that may shape many of their careers moving forward.
Prevention services scaled back
Although clinical screening and prevention services were scaled back during the pandemic based on federal and regional guidance, we recognize the importance of quickly bringing these services back online for our patients, and we are working to do so in locations all over Houston.
This will be enhanced by ensuring we can ease the anxieties that healthy people have about accessing hospitals—fearing the risks of contracting COVID-19 at a health care facility to be far greater than at the grocery store or other public venues where the sick do not congregate.
Throughout the pandemic, social media has played an important role in continuing to communicate prevention messages. Those stories and tips have served as a reminder that there always are steps that can be taken to reduce cancer risk.
Recovery creates opportunities


If our initial response was like turning off a light switch, our recovery is like using a series of dimmer switches with the ability to adjust up and down based on COVID-19 case volumes in the area.
The principles of our recovery are that it be strategic, stepwise, fact-based and data-driven. It is clear that COVID-19 will be with us for years, and we must move forward understanding and accepting this new reality. It is essential that we continue our commitment to cancer patients and clinical trials while fully integrating protection for our patients and our employees in our recovery plan.
We know that we cannot just open the doors. We have to create social distancing capacity and redesign clinics, and we even have to rethink long-term changes to how individuals enter the institution. We also must maintain a disciplined way to preserve at least 15% of our beds, as required by our state’s governor, for the second surge of COVID-19, which may be upon us now.
The goal that we initially set for full reopening to normal operating volumes was Nov. 1. Already, we are far outpacing our goal. We are learning from our experience and transforming operations and medical practice, moving forward in dramatic ways that extract elements from our draft strategic plan and allow for accelerated implementation.


So, what has changed, and how are we making such swift progress?
We first looked at what has been impacted by the decrease in patient volumes. We rescheduled appointments and got our surgical services back to full capacity. We also found that we can make improvements to our surgical scheduling, which is making a positive impact.
We have focused initially on service lines that offer high-impact clinical trials, including Lymphoma and Myeloma with our CAR T and CAR NK trials. We also have explored ways to improve our patient navigation process.
Regulatory changes have allowed for increased virtual care and transformation. Virtual care is one example of an initiative that was included in our draft strategic plan. Traditionally, getting an effort like that implemented would take three years, whereas in a crisis we made it happen in three weeks. Already, virtual care has proven impactful for patients and providers, with nearly 20,000 appointments having been completed using our platform.
We have increased the frequency of working with our patients to establish and document goals of care based on each individuals’ preferences and beliefs. This ensures that there is complete alignment and mutual understanding of the fundamental aspects of the pace of disease, changes in prognosis, and patient and family goals of care. While we always strive for a cure, compassionate care aligned with the patient’s goals is of greatest importance.
We also are developing plans to continue allowing a portion of our employees to work remotely—even after the current concerns have been alleviated. This concept would have been unheard of just a few months ago, but now we are aiming for a substantial proportion of our workforce to be remote for increased efficiency, effectiveness, work-family balance and a reduced carbon footprint.
We also know that our workforce has more than 6,000 children under the age of 14 whose needs must be factored into our planning if onsite school classes are not offered in the fall.
In our research areas, our timely recovery is critical as we know clinical trials often are a patient’s best treatment option and that resuming activity maintains grants and other external funding sources.
Additionally, we want to get our researchers back to work as that has a positive impact on their projects, on their career growth opportunities and their well-being.
Our research recovery is happening in phases: first, preparing and getting buildings back online; second, returning to work in shifts to enable extra safety precautions; third, full capacity and functioning within the “new normal,” and last, at some undetermined date in the future, business as usual allowing for in-person seminar series and business travel.
We currently are in the second phase of our recovery, with great momentum carrying us forward. We recognize that the pace of our phases must be dovetailed with the evolution of the secondary peaks in the pandemic and the development of a safe and effective vaccine(s).
We are cognizant of the reality that it is not the date of the availability of a vaccine that is a pivot point, but that it is the date that 100% of our workforce has developed antibodies through SARS-CoV-2 or via vaccination.
Overcoming financial hurdles
Like most hospitals around the country that have been impacted by COVID-19, MD Anderson is projecting a financial loss in fiscal year 2020.
Currently, we are well positioned to overcome these financial challenges based on prudent decision-making and the institution’s overall financial strength at the start of the pandemic.
That said, we have taken additional steps to respond to financial results and to ensure the long-term health of the institution. Some of those steps have included the implementation of a hiring freeze until the end of the fiscal year (Aug. 31, 2020), elimination of incentives, the reduction or elimination of overtime, the reduction of contractor and consulting agreements and the deferment of some facility and IT projects. Business travel also remains on hold due to the virus.
Our team effort has helped minimize the financial loss for the institution, and, as one of the largest employers in Houston, it has ensured our ability to remain firmly committed to job security for our workforce. We strongly believe that we have a responsibility to protect our employees and their families during a national recession and economic headwinds in Texas related to energy markets and crude oil prices.
Coming together to emerge stronger, safer and better


Our collective focus has been on our three goals of protecting our patients, ensuring the safety of our employees and minimizing the impact on our community.
By working to accomplish our goals, we have been able to ensure the safety of our patients and our people while continuing our important work to end cancer. We recognize the formidable role we play in advancing cancer discoveries through research, in educating and training future health care providers and in preventing cancer before it starts through screening efforts and early detection.
While much has changed, we never have lost focus on our mission. We have come closer together as an institution, we have learned so much, and we remain committed to a safe and complete recovery.
There is no doubt that this has been a very difficult time for everyone involved—patients, their loved ones, health care providers, researchers, and our entire community. We are committed to seeing those patients who need safe cancer care, to accelerating discovery through our research efforts and to ensuring availability of cutting-edge clinical trial options for our patients.
We stand with our researchers and are committed to ensuring they have the support they need to continue doing groundbreaking work. We stand with our physicians, our advanced practice providers, our nurses, our patient transporters, our entry screeners and all of our employees on the frontlines of this crisis.
And we stand with all of you—our peers in the cancer community. We have an important role to play in ensuring the health of our nation and in caring for cancer patients, many of whom will need us now more than ever. I applaud all of you for the work you have done during COVID-19.
And as I tell my team of 22,000 cancer fighters: Together, we will emerge stronger, safer and better, forever.













