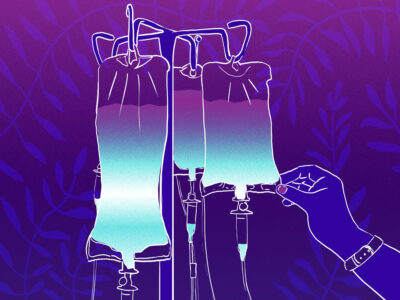
Hospitals and radiology clinics are rationing—of all things—intravenous contrast agents used for computed tomography imaging.
“Jim Allison: Breakthrough,” a documentary released in 2019, tells the story of 2018 Nobel Prize winner Jim Allison’s quest to cure cancer and the development of ipilimumab.
The same House appropriators who, with bipartisan resolve, oversaw years of dramatic funding increases for NIH expressed equally bipartisan misgivings about President Joe Biden’s proposal to boost funding for the Advanced Research Projects Agency for Health while giving NIH a meager raise—and cutting funds for NCI.
The COVID-19 pandemic has demonstrated that real-world data is an indispensable tool healthcare professionals should use to rapidly respond to emerging gaps in care delivery.
In a panel discussion this week, five leaders in oncology proposed an action plan for tackling cancer health disparities and enhancing health equity.
The Cancer History Project is focusing on health equity and the evolution of ethics in clinical trial research during the month of May.
We wish to applaud the sentiments expressed in the recent article in The Cancer Letter titled “Oncologists, advocates, FDA call for an end to MTD and ‘more is better’ era in cancer drug dosing,” but also raise several concerns to be addressed as this initiative moves forward.
FDA is asking sponsors of all investigational medical products to focus on including diverse patients throughout the clinical development process.
Getting the dose right is not a mere formality but a fundamentally important first step that can mean the difference between success and failure of a drug development program, Richard Pazdur, director of the FDA Oncology Center of Excellence, said at a workshop focused on the issue of dose optimization.
Before any strategy can be formulated for next year’s appropriations, cancer groups must confront the formidable challenge of figuring out how much of President Joe Biden’s vision for cancer research is realistic.