The FDA Oncologic Drugs Advisory Committee recommended against approval of the Novartis agent Farydak (panobinostat), a pan-deacetylase inhibitor, for the indication of previously treated multiple myeloma when used in combination with bortezomib and dexamethasone.
FDA approved Avastin in combination with chemotherapy for the treatment of women with platinum-resistant, recurrent ovarian cancer.
This week the Centers for Medicare & Medicaid Services issued a proposed rule stating that the scientific evidence was sufficient to support reimbursement for counseling on the risks and benefits of lung cancer screening as well as lung cancer screening with low dose computed tomography in high risk individuals and once per year. CMS will pay for such services when provided to beneficiaries at high risk for lung cancer and when provided by physicians and centers with specific qualifications.
CT screening of the lungs of current and former heavy smokers is about to become a Medicare benefit.
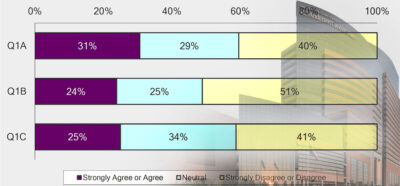
After three very similar surveys yielded results that pointed to disaffection and disenfranchisement on the part of the faculty at MD Anderson, the UT System officials said they expect a “renewed, constructive and collaborative effort” to address the problems.
Over the past two years, four separate surveys attempted to gauge the level of faculty morale and satisfaction at MD Anderson Cancer Center.
Charles Bennett, an oncologist and cancer researcher whose work focuses on adverse events caused by pharmaceutical products, settled a federal complaint brought by a whistleblower alleging irregularities in the management of R01 research grants administered by Northwestern University.
The nation's academic cancer centers are a national resource that will increase in value as remarkable changes continue in biomedical research, cancer care, and health policy.
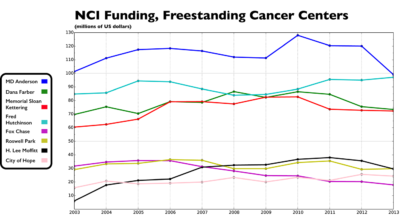
The Cancer Letter asked leaders of cancer centers, professional societies, and science advocacy organizations to comment on declining levels of NIH and NCI funding at freestanding cancer centers and selected academic institutions that include cancer centers.
The ten-year period of erosion that followed the doubling of the NIH budget has hit some research institutions harder than others.