APTOSE BIOSCIENCES Inc. joined the Beat AML collaboration, developed by The Leukemia & Lymphoma Society and the Knight Cancer Institute at Oregon Health & Science University.
LAURA BROD was named CEO of GeneSegues Therapeutics. Brod is an at-large member of the University of Minnesota Board of Regents and is chair of the university's Audit Committee. She was a member of the Minnesota House of Representatives from 2002 to 2010, during which time she served as assistant majority leader.
RONAN SWORDS received the Pap Corps Endowed Professorship in Leukemia at the University of Miami Sylvester Comprehensive Cancer Center. Swords is assistant professor of medicine and director of the Leukemia Program at Sylvester.
UNIVERSITY HOSPITALS Case Medical Center and UH Seidman Cancer Center selected GO Clinical Workbench developed by GenomOncology for workflow management of next generation sequencing data.
QIAGEN N.V. and Astellas Pharma Inc. will collaborate to develop and commercialize companion diagnostics paired with Astellas drugs for use in cancer and other diseases.
FDA approved the expanded use of Lymphoseek (technetium Tc 99m tilmanocept) injection for lymphatic mapping in solid tumors, and adding sentinel lymph node detection for breast cancer and melanoma to the approved indications.
Charles Bennett, an oncologist and cancer researcher whose work focuses on adverse events caused by pharmaceutical products, settled a federal complaint brought by a whistleblower alleging irregularities in the management of R01 research grants administered by Northwestern University.
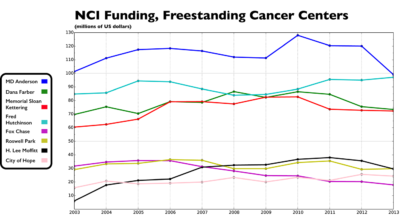
The nation's academic cancer centers are a national resource that will increase in value as remarkable changes continue in biomedical research, cancer care, and health policy.
The Cancer Letter asked leaders of cancer centers, professional societies, and science advocacy organizations to comment on declining levels of NIH and NCI funding at freestanding cancer centers and selected academic institutions that include cancer centers.
The ten-year period of erosion that followed the doubling of the NIH budget has hit some research institutions harder than others.