MD Anderson Cancer Center President Ronald DePinho and his executive leadership declined to meet with the investigation committee dispatched by the American Association of University Professors to his institution Thursday.
Is the new National Clinical Trials Network set up for success or heading for failure?
It's possible that molecular testing is doing a lot of good, pinpointing cancer therapies that are most likely (or least likely) to work.
Ethicon, the Johnson & Johnson subsidiary that manufactures nearly three-quarters of laparoscopic power morcellators on the market, has requested a withdrawal of the controversial devices.
There was no formal consensus on either an outright ban on power morcellators or issuance of a “black box” warning label.
The American Association of University Professors has authorized a formal investigation of MD Anderson Cancer Center, a move that could result in censure.
NCI took another step toward adopting a new formula for determining the size of cancer center support grants, with the National Cancer Advisory Board accepting a report from a working group that has been working on the problem since the fall of 2012.
On Oct. 17, 2013, a surgical instrument called a power morcellator tore into the uterus of Amy Reed, an anesthesiologist at Beth Israel Deaconess Medical Center, pulverizing what were believed to be benign fibroids.
Some of the questions that landed the AstraZeneca drug Olaparib (lynparza) before the FDA Oncologic Drugs Advisory Committee were classic:
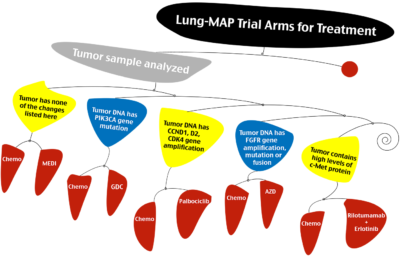
SWOG earlier this week started to accrue patients to Lung-MAP, a clinical trial for second-line treatment of non-small cell lung cancer.