Earlier this year, FDA announced that it would be initiating a voucher program aimed at accelerating review time for applications that are deemed to be advancing U.S. “national priorities” (The Cancer Letter, June 20, 2025).
A court filing by Thomas Nagy Jr., deputy assistant secretary for Human Resources and chief human capital officer at HHS, claims that his department can proceed with the 982 RIFs it issued two weeks ago, despite a judge’s temporary order freezing the notices issued by two dozen federal agencies since the government shutdown that began on Oct 1.
With the start of open enrollment less than one week away, millions of Americans are getting their first look at the sharp increases many will pay next year if Congress fails to extend the enhanced premium tax credits that have expanded healthcare access to over 13 million people since 2021.
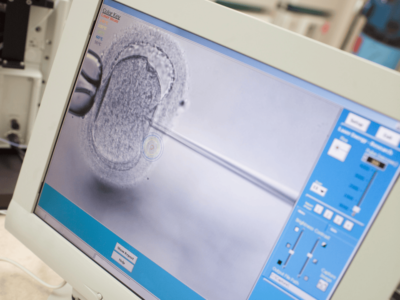
President Donald Trump has announced a deal with pharmaceutical company Merck KGaA and its U.S. subsidiary, EMD Serono, to sharply reduce the cost of certain fertility drugs used for in-vitro fertilization, or IVF.
Millions of Americans with Affordable Care Act marketplace health insurance will face higher costs next year if Congress doesn’t extend enhanced premium tax credits that have made the plans more affordable for low- and middle-income enrollees.
A federal judge issued a temporary restraining order on Oct. 15, ordering the Trump administration to reverse the wave of layoffs that the administration is using as a pressure tactic intended to force the Democrats to accept cuts to popular health programs and reopen the government.
As of Oct. 1, Medicare no longer covers telehealth visits.
A report by the American Society of Clinical Oncology points to a growing challenge in cancer care access driven by a widening gap between the number of available oncologists and increasing patient demand.
President Donald Trump had threatened pharmaceutical companies with a 100% tariff, with an ultimatum set for Oct. 1.
Jim O’Neill, acting director of the Centers for Disease Control and Prevention, announced that he has signed off on a slew of recommendations that change the childhood vaccination schedule.