Latest Stories
A coalition of 86 organizations is urging Congress to pass the Clinical Treatment Act, a bill introduced in January that would guarantee coverage of the routine care costs of clinical trial participation for Medicaid patients with a life-threatening condition.
By Claire Dietz
Conversation with The Cancer Letter
A report by the National Academy of Sciences, Engineering, and Medicine recommends that HHS and other federal agencies form an independent organization that would rely on complex adaptive sysytems to direct cancer control in the U.S.
By Matthew Bin Han Ong and Claire Dietz
The National Academies of Sciences, Engineering, and Medicine has proposed that HHS form an independent agency or consortium to direct a national strategy for cancer control in the United States.
By Claire Dietz and Matthew Bin Han Ong
The NCI Board of Scientific Advisors has approved 23 new and reissue concepts: five Requests for Applications/Requests For Proposals and 18 PARs, program announcements reviewed in the institute.
By Claire Dietz
Free
Emory University has terminated two NIH-funded faculty members at the Department of Genetics for failing to disclose foreign sources of funding and the extent of their involvement with institutions in People's Republic of China.
By Claire Dietz
Free
Project ECHO, a service that provides physicians in rural areas with access to multidisciplinary expertise, will soon announce partnerships with four cancer centers—Dana-Farber Cancer Institute, Mayo Clinic Cancer Center, University of New Mexico Comprehensive Cancer Center, and Yale Cancer Center.
By Claire Dietz
The Texas Senate May 10 passed a measure that would provide new funding for the Cancer Prevention and Research Institute of Texas.
By Claire Dietz
The House Committee on Appropriations May 8 approved a spending bill that gives NIH $41.1 billion, a $2 billion increase over the current level. The bill passed on a vote of 30 to 23.
By Claire Dietz
The Subcommittee on Labor, HHS and Education of the House Committee on Appropriations April 30 marked up a spending bill that gives NIH $41.1 billion, a $2 billion increase over the current level.
By Claire Dietz and Matthew Bin Han Ong
The Texas House of Representatives approved two measures that would give the Cancer Prevention and Research Institute of Texas an additional $3 billion to continue operations, and allow the institute to continue funding grants beyond fiscal year 2023.
By Claire Dietz
Free
The NCI Board of Scientific Advisors approved eight new Requests for Applications, Requests for Proposals, and PAR—a program announcement reviewed in the institute—concepts at a meeting March 25, including three new Cancer Moonshot concepts.
By Claire Dietz
Pharmacy benefit managers, like climate change, germs and, possibly, God, are all around, and if we could clear a few hours, it would be good to learn enough about them to sustain a cocktail party conversation.
By Claire Dietz and Paul Goldberg
The Cancer Prevention and Research Institute of Texas—the second largest publicly-funded granting organization for cancer after NIH—may receive $3 billion more to stay open past its current sunset date in 2023.
By Claire Dietz and Matthew Bin Han Ong
Conversation with The Cancer Letter
Advocates of renewing the Cancer Prevention and Research Institute of Texas argue that the agency is a good investment for the state.
By Claire Dietz and Matthew Bin Han Ong
Free
President Donald Trump has proposed to cut $4.7 billion from the NIH budget, with almost $900 million coming out of NCI during the fiscal year 2020.
By Claire Dietz and Matthew Bin Han Ong
Conversation with The Cancer Letter
The number of cases of HPV+ cervical precancers has dropped by 21 percent from 2008 to 2014, according to a survey by the Centers for Disease Control and Prevention.
By Claire Dietz
Free
In his State of the Union address Feb. 5, President Donald Trump said he plans to ask Congress for $500 million over 10 years to fund pediatric cancer research—an amount Speaker of the House Nancy Pelosi (D-CA) said is insufficient.
By Claire Dietz
Free
Earlier this week, the Senate Committee on Finance convened what is likely to be the first in a series of hearings focused on the rising costs of prescription drugs, oncology drugs among them.
By Claire Dietz
Free
Last year, FDA approved 19 applications for new cancer drug and biologics as well as 38 supplemental indications and four biosimilars, agency officials said.
By Claire Dietz and Matthew Bin Han Ong
The NCI Board of Scientific Advisors approved five Requests for Applications, as well as two Cancer Moonshot concepts, at a joint meeting with the National Cancer Advisory Board on Dec. 4, 2018.
By Claire Dietz
Conversation with The Cancer Letter
A SWOG study estimates that implementation of findings from that group's clinical trials has added 3.34 million years to the lives of cancer patients in the 60 years since its founding in 1956.
By Claire Dietz
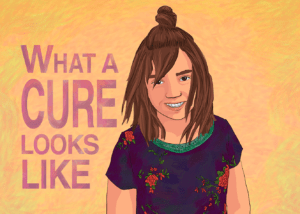
“If you want to see what a cure looks like, you already have,” said Tom Whitehead as his daughter Emily joined him at the lectern at the public hearing of the FDA Oncologic Drugs Advisory Committee July 12.“She's standing right beside me.”
By Claire Dietz and Paul Goldberg
If the June 22 Senate appropriations hearing is an indication, President Donald Trump will encounter considerable difficulty in accomplishing his stated goal of cutting the NIH budget by 21 percent in fiscal 2018.
By Claire Dietz
Resolving a three-year-long court fight, the U.S. Supreme Court June 12 ruled that Sandoz can commence marketing of its biosimilar white blood cell growth factor immediately after getting FDA approval.
By Claire Dietz
Tobacco use should be addressed as a social justice issue, according to a recent report by Action on Smoking and Health.
By Claire Dietz