ODAC member Brent Logan, professor of biostatistics at the Medical College of Wisconsin, said he didn't have confidence in the PFS advantage seen in Study 19.
Some of the questions that landed the AstraZeneca drug Olaparib (lynparza) before the FDA Oncologic Drugs Advisory Committee were classic:
The Health Resources and Services Administration said it stands by its interpretation of the Affordable Care Act orphan drug exclusion, despite a recent court ruling that challenged its authority to engage in legislative rulemaking.
BALTIMORE—Constructed in Germany, shipped to the port of Baltimore, and driven through downtown during the night, the 90-ton cyclotron arrived at the University of Maryland's Proton Treatment Center.
The Cancer Letter asked David Wholley, director of the Foundation for the National Institutes of Health Biomarker Consortium, to explain the novel scientific and administrative structure of Lung-MAP.
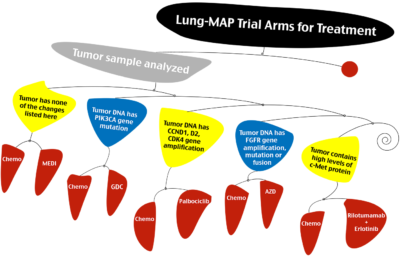
Registration Trial DesignLung-MAP is designed to provide a fast, efficient path to full approval, said Roy Herbst, co-chair of the trial's steering committee and co-chair of its executive operations group. The idea is to get drugs to patients throughout the community, going seamlessly from a phase II portion to phase III, with all patients counting toward registration.
SWOG earlier this week started to accrue patients to Lung-MAP, a clinical trial for second-line treatment of non-small cell lung cancer.
The number of high school boys who smoke cigars—16.5 percent—is now on par with cigarette use, said the Centers for Disease Control and Prevention.
Cancer survivors face higher medical costs and productivity losses when compared to people without a cancer history, according to a CDC study published June 13.
The Centers for Medicare and Medicaid Services have another six months to decide whether to cover low-dose computed tomography screening. Yet, proponents of screening seem unwilling to take the chance that Medicare coverage would be restrictive.