The American Association of University Professors sent a letter to Ronald DePinho, president of MD Anderson Cancer Center, urging the reinstatement of two faculty members who were denied tenure renewal without stated reasons.
NCI: NCTN Makes “Amazing Trials” Possible“It is important to note that the $151 million does not capture the many other functions that NCI provides in support of the NCTN and the entire clinical trials enterprise,” Garrett said. “A few of these key centralized functions include the Cancer Trials Support Unit (CTSU), a 'one-stop shop' for the groups to access all trials, a Central Institutional Review Board (CIRB) to eliminate the need for local IRB approvals, tumor banking for each group, and ancillary studies funded to support biomarker and quality of life research.
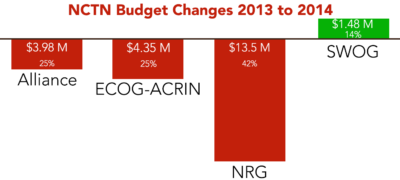
The budgets of operations and statistical centers of adult clinical trials groups were cut by about $20.4 million, group chairs say.
MD ANDERSON CANCER CENTER honored 16 junior faculty members with the first R. Lee Clark Fellow awards. The award was established to recognize outstanding work by junior faculty members.
PETER BACH's account of his wife's death from breast cancer—"The Day I Started Lying to Ruth"—was published in New York Magazine May 6.
FDA granted accelerated approval to Zykadia (ceritinib) for patients with a certain type of metastatic non-small cell lung cancer.
FDA granted orphan drug designation to ADXS-HPV for the treatment of stage II-IV invasive cervical cancer. ADXS-HPV is an immunotherapy drug candidate, developed by Advaxis Inc., which is designed to target cells expressing the HPV gene E7. Expression of the E7 gene from high-risk HPV variants is responsible for the transformation of infected cells into... […]
KRISTIN DARBY was named chief information officer of Cancer Treatment Centers of America. She will be the principal architect of the organization's Information Services function, including all clinical and non-clinical hardware and software applications, data infrastructure, warehousing and security, informatics, and system-wide technology support services.
THE MELANOMA RESEARCH ALLIANCE and L'Oreal Paris launched It's THAT Worth It, a campaign to support melanoma research.
THE JOHNS HOPKINS Kimmel Cancer Center received $10 million from Under Armour to help build a breast health center. The gift comes from the company's Power in Pink Campaign, and the facility will be named the Under Armour LiveWell Center.