
Cover Story
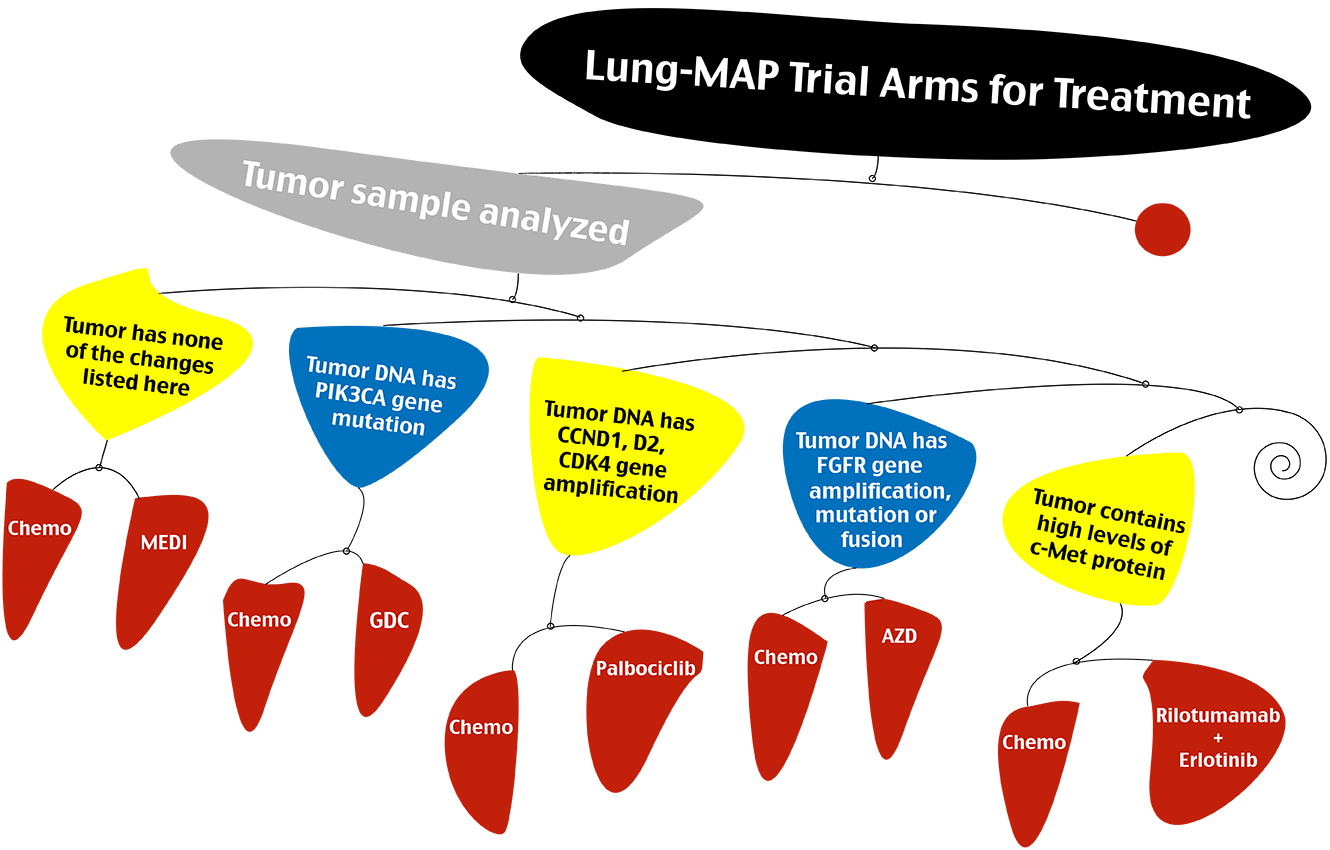
SWOG earlier this week started to accrue patients to Lung-MAP, a clinical trial for second-line treatment of non-small cell lung cancer.
By Tessa Vellek
In Brief


Trending Stories
- The Directors: Tom Lynch and Skip Burris on how NIH funding cuts imperil biopharma innovation—and cost patient lives
In a time of uncertainty, “react to the knowns, not the fear” - How MD Anderson and Texas Children’s made plans to build a $1 billion pediatric cancer hospital—one of the world’s largest
- In the Headlines: MD Anderson’s Pisters on doing “something gigantic for pediatric cancer”
- The faces of RIF: Staff members of NCI’s dissolved communications team gather for a farewell group photo
- As cancer scientists, we must change how we engage with the public on the impact of NIH cuts
What the scientific method obscures - In weekly vigils, current and former NIH staff grieve the impact of Trump cuts
The Saturday gatherings at NIH’s Metro station are part graveside service, part street theater













