Changes in the NCI Clinical Trials System
NCI has launched a pilot study to assess whether assigning cancer patients treatment based on the genetic characteristics of their disease can improve outcomes for patients with advanced metastatic solid tumors.
By Matthew Bin Han Ong


Two years ago, NCI officials made a promise to increase the budget of the cooperative groups program by $25.6 million.


The chairs of the adult clinical trials groups that make up the NCI National Clinical Trials Network said in a letter that recent budget cuts have triggered a “crisis” in clinical research.


Following an explosion of criticism, NCI said funding for community oncology clinics would not be interrupted.


NCI officials said they plan to hold a series of meetings with clinical trials group chairs and group financial officers in order to fine-tune the new National Clinical Trials Network.


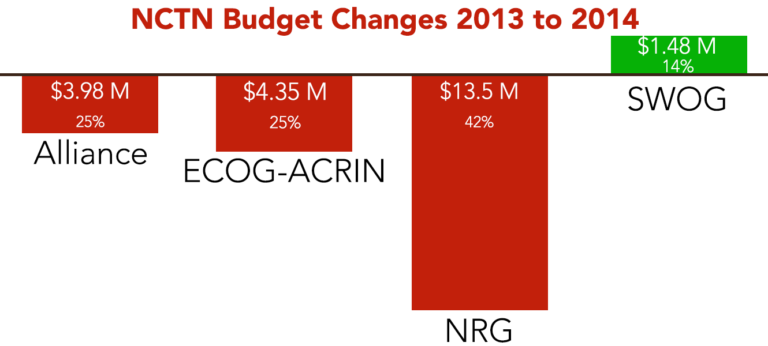
The budgets of operations and statistical centers of adult clinical trials groups were cut by about $20.4 million, group chairs say.

As funding issues surrounding the NCI's new National Clinical Trials Network (NCTN) have been a prominent topic of recent news and discussions, to help inform the cancer research community, we are taking this opportunity to share data regarding NCTN funding to the Children's Oncology Group (COG).
CHICAGO—The consequences of diminishing federal support for cancer research can be measured in the abstracts presented at the 50th annual meeting of the American Society of Clinical Oncology that concluded earlier this week.


SWOG earlier this week started to accrue patients to Lung-MAP, a clinical trial for second-line treatment of non-small cell lung cancer.


NCI launched another in a series of targeted treatment trials referred to as the AdjuvantLungCancer EnrichmentMarkerIdentification andSequencingTrial.
Is the new National Clinical Trials Network set up for success or heading for failure?


NCI has launched a pilot study to investigate the molecular factors of tumors associated with exceptional treatment responses of cancer patients to drug therapies,The Exceptional Responders Initiative seeks to identify the molecular features of tumors that predict whether a particular drug or class of drugs will be beneficial.
Top NCI officials made an unusual assurance that the reorganization of clinical trials cooperative groups isn't a “prelude to reducing the commitment of the NCI to clinical trials-based research.”





