In Their Own WordsThese comments by MD Anderson faculty and staff members were selected from an 835-page document. They appear exactly as they were keyed in, with no editing. The document was obtained under the Texas Public Information Act. Names and titles of MD Anderson officials were redacted.
“Much to Be Proud Of”In an email to employees, titled, “A BIG thank you,” DePinho lauded the high response rate, saying that the survey scores were favorable.
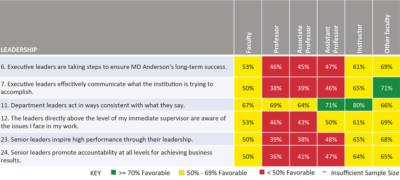
In a long-awaited survey of employees at MD Anderson Cancer Center, faculty members show a significant drop in approval scores for the administration's executive leadership, in comparison with the last time the survey was administered in 2012.
The Translational Genomics Research Institute and George Mason University announced a strategic research alliance May 6.
The 340B drug discount program is causing a rise in the costs of treating cancer patients, according to a new report.
AAUP: “Term Tenure” is “Oxymoronic”MD Anderson's “term tenure” system is not tenure, AAUP said.
No Justification Provided AAUP Demands Reinstatement of Faculty Denied Tenure Renewal at MD Anderson
The American Association of University Professors sent a letter to Ronald DePinho, president of MD Anderson Cancer Center, urging the reinstatement of two faculty members who were denied tenure renewal without stated reasons.
NCI: NCTN Makes “Amazing Trials” Possible“It is important to note that the $151 million does not capture the many other functions that NCI provides in support of the NCTN and the entire clinical trials enterprise,” Garrett said. “A few of these key centralized functions include the Cancer Trials Support Unit (CTSU), a 'one-stop shop' for the groups to access all trials, a Central Institutional Review Board (CIRB) to eliminate the need for local IRB approvals, tumor banking for each group, and ancillary studies funded to support biomarker and quality of life research.
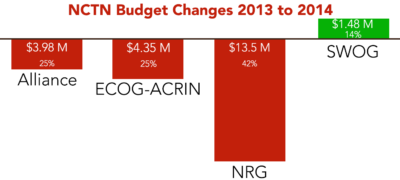
The budgets of operations and statistical centers of adult clinical trials groups were cut by about $20.4 million, group chairs say.
A study by a conservative think tank found large differences in performance of the FDA divisions, with oncology demonstrating the agency's fastest time from application submission to approval.