Rahul Jandial has received a $1.35 million grant from the Department of Defense Breast Cancer Research Program to support his laboratory research into leptomeningeal disease.
In an unprecedented use of real-world data, researchers at the University of Pennsylvania and Flatiron Health have determined that oncologists are responding quickly to label restrictions announced by FDA.
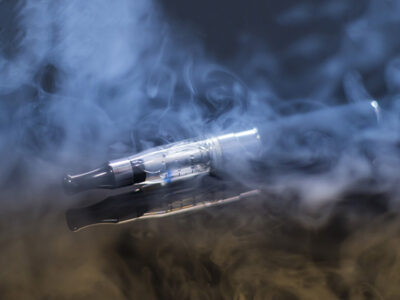
E-cigarette use is on the rise among young adults, but overall combustible cigarette use among teens is continuing on a downward trend, recent studies show.
In the first regulatory action of its kind, FDA and drug approval authorities in Canada and Australia simultaneously approved a new treatment for patients with endometrial carcinoma.
Philip J. Stella, the principal investigator of the Michigan Cancer Research Consortium NCI Community Oncology Research Program site in Ann Arbor, won the 2019 Harry Hynes Award.
The game of musical chairs involving topmost jobs in oncology has gathered speed and expanded beyond FDA and NCI, pulling in premier positions at MD Anderson Cancer Center and Memorial Sloan Kettering Cancer Center.
Soon after the Chernobyl accident, while caring for victims at Moscow's Hospital No. 6, I commented: “In a nuclear age, an accident anywhere is an accident everywhere.”
It is estimated that, each year, as few as 2–3% of cancer patients enter clinical trials. In part I of this series, we discussed four key barriers to participation: physician barriers, protocol barriers, research team barriers, and insurance barriers. In part II, we will look at solutions to these barriers and how to implement them in the clinical setting.
This story is part of our 2019 Summer Reading Series. You can read the whole series here.
This story is part of our 2019 Summer Reading Series. You can read the whole series here.