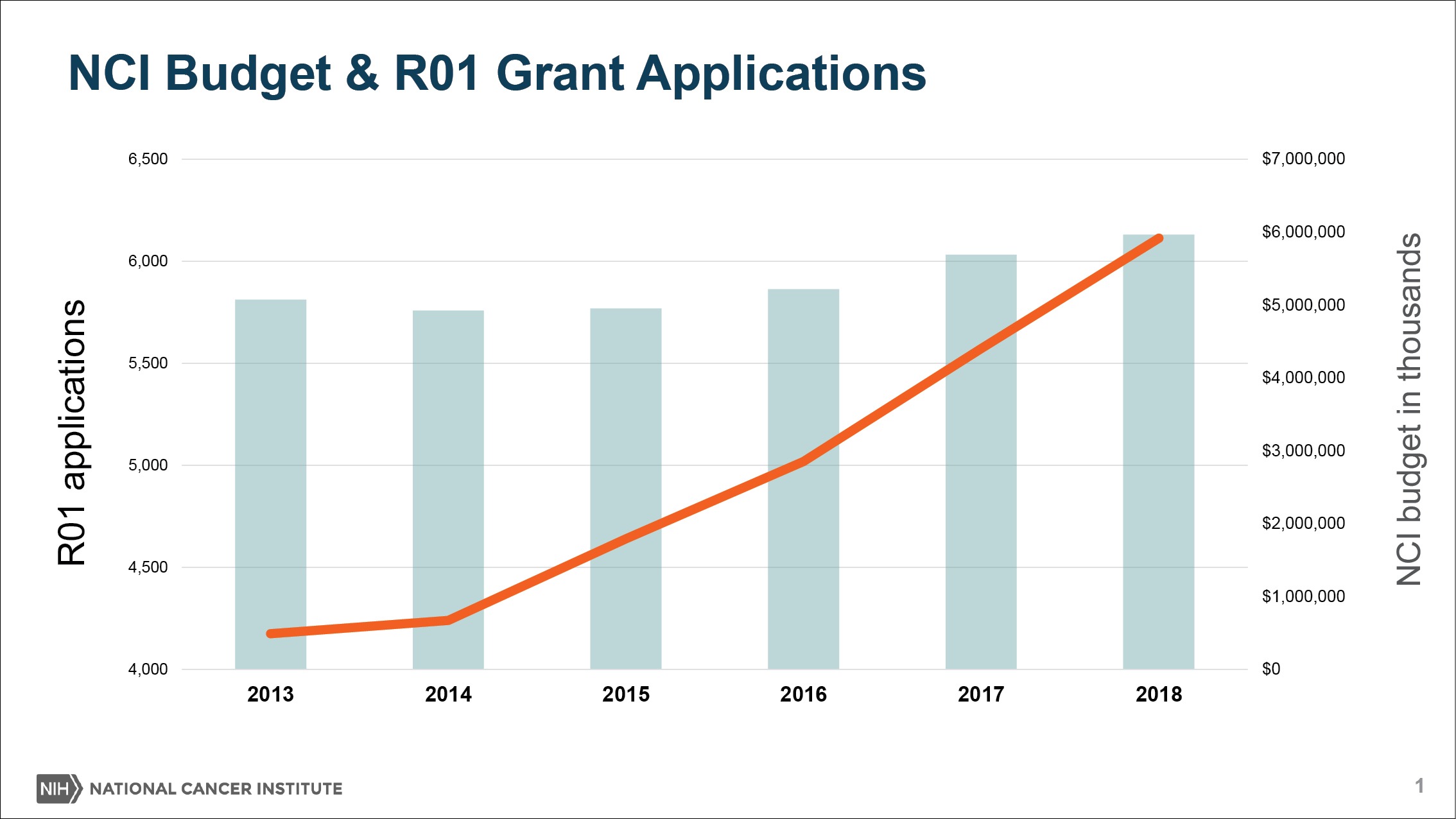
In recent years, NCI spending on extramural research has been following the same trajectory as Congressional appropriations for the institute: up, up, up.
This expansion resulted from a strategy by NCI Director Ned Sharpless and his recent predecessors to grow investigator-initiated research and the cancer centers. Now, growth has slowed, as out-year grant payments place a limit on the number of new grants NCI is able to issue in 2019.
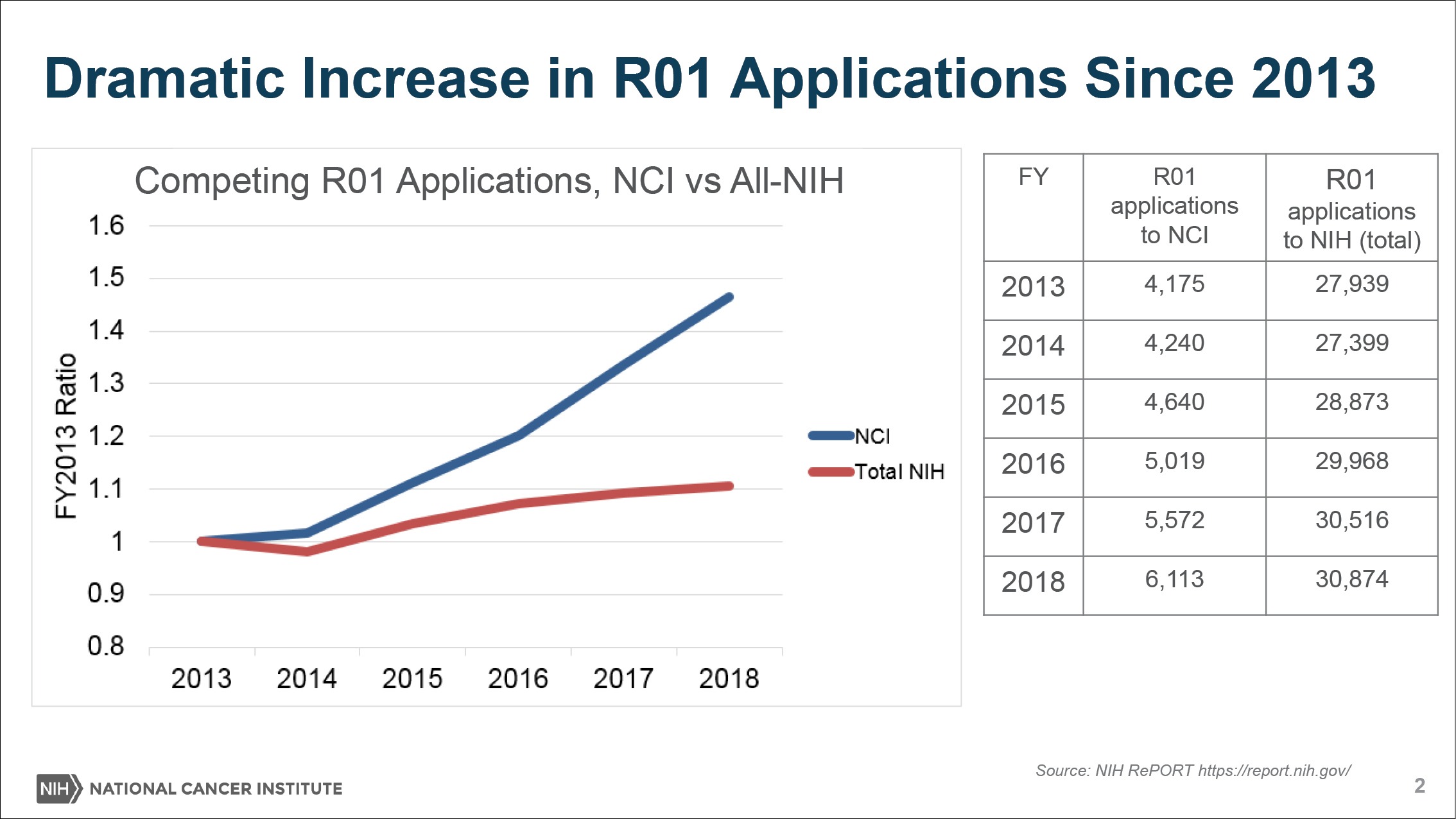
On top of that, NCI is contending with a rising number of grant applications, propelled by the availability of funds and new possibilities in the field. As the denominator—the pool of applicants—rises, the applicants’ success rates, or paylines, would plummet.
With fiscal pressures rising, something had to give, and NCI Director Sharpless recently opted to trim the institute’s intramural spending in order to maintain the paylines.
Since making this choice, forum after forum, Sharpless has been explaining a notion that some might regard as counterintuitive: that NCI has to take austerity measures in the midst of a years-long stretch of healthy appropriations.
If we didn’t make adjustments for fiscal year 2019— modest reductions to operating budgets for NCI divisions, reducing new, nonmodular R01s by an additional two percent, and reducing many noncompeting grants by 3 percent — paylines would be lower still.
Sharpless started his Explaining Tour at the meeting of the National Cancer Advisory Board and the Board of Scientific Advisors last December (The Cancer Letter, Dec. 7, 2018). He returned to the lectern on Jan. 25, hosting a Town Hall at NCI to explain his rationale for the cuts.
A webcast of his remarks is posted here.
Earlier this week, Sharpless sat down for a chat with The Cancer Letter as well.
In fiscal 2018, Sharpless increased funding for the Research Project Grant pool by $146 million, providing the largest increase to the pool since 2003 (The Cancer Letter, March 30, 2018).
These increases on the extramural side come at a price: all internal operating budgets of NCI divisions, offices and centers are subjected to a 5-percent cut, across-the-board in FY19.
“Because of the success of cancer research, we’re having so many people in our field and we’re getting so many great ideas. We’ve had this very sharp uptick in the number of grant applications we’re receiving for certain kinds of grants like the R01 award,” Sharpless said to The Cancer Letter. “Grants are up nearly 60 percent since 2009 and nearly 50 percent since 2013.
“The NCI leaders agree that we want these grants coming in and we’d like people to send their ideas for funding.We want to try to support them and really support innovation to the extent possible. And I think there’s buy-in on why we have to do this.
“Any cut at all is different from the status quo and it is causing some hardship. I think that no one’s enthusiastic about this, but I think everybody understands the issue.
“I think if we didn’t make adjustments for fiscal year 2019—modest reductions to operating budgets for NCI divisions, reducing new, non-modular R01s by an additional two percent, and reducing many noncompeting grants by 3 percent—paylines would be lower still.”
In an email sent to members of NCI’s advisory committees in December, NCI Director Ned Sharpless summarized the budgetary changes:
Make internal budget adjustments across NCI, including all divisions, offices and centers, which will operate at 95 percent of FY 2018 levels.
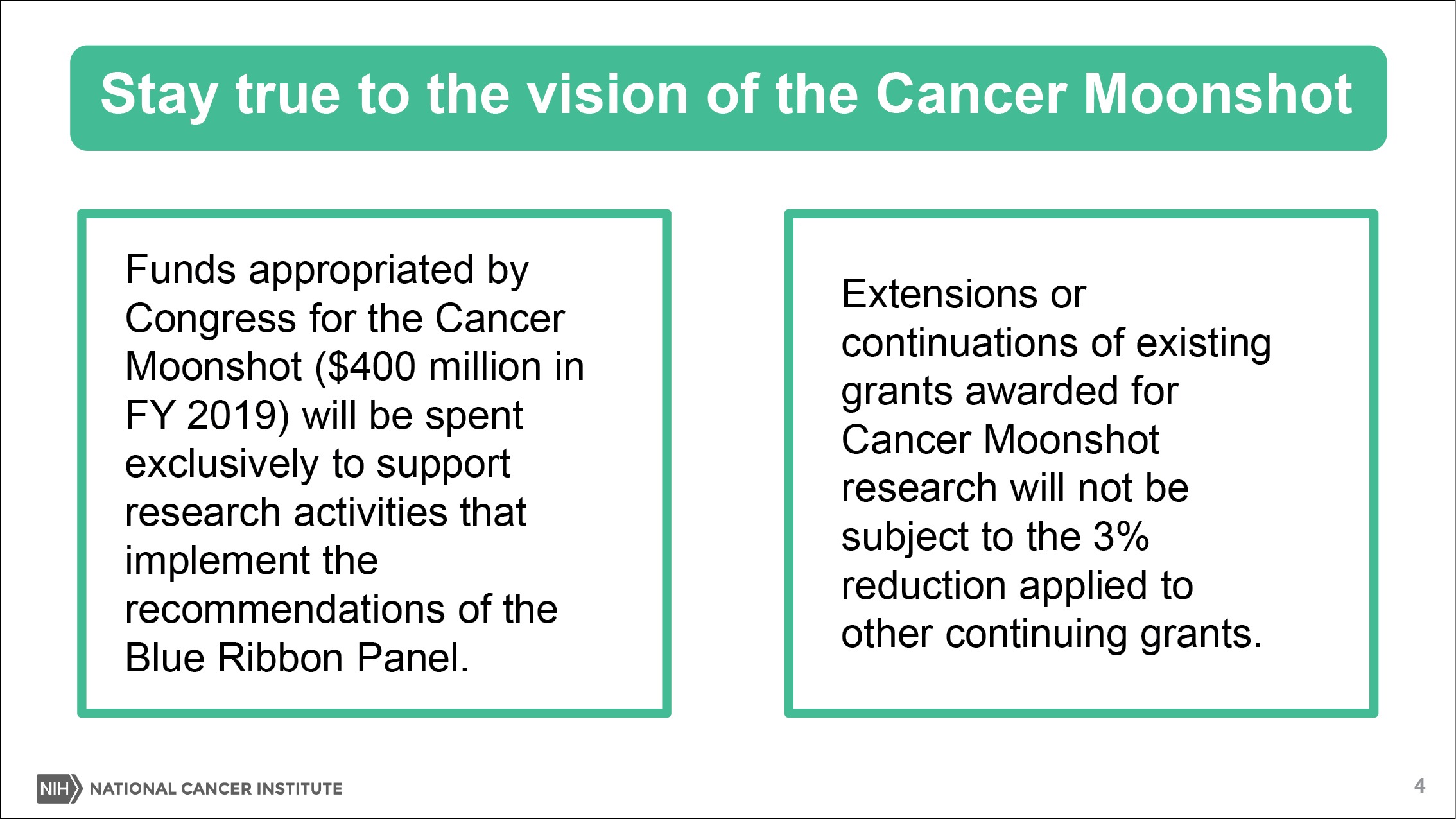
Fund non-competing grant continuations at 97 percent of the committed level, with the exception of Cancer Center Support Grants, Cancer Moonshot grants, and NRSA awards.
Change the funding policy reduction to competing new and renewing grants (Type 1s and Type 2s) by 2 percent compared to FY 2018, from 17 percent to 19 percent.
Fund new grants up to and including the 8th percentile.
Maintain the [early-stage investigator] payline at 14 percent or better.

Sharpless spoke with Paul Goldberg, editor and publisher of The Cancer Letter, and Matthew Ong, a reporter with The Cancer Letter.
Paul Goldberg: How is NCI doing this year? Is the shutdown affecting you?
Ned Sharpless: NCI is open. We received our funding, as you know, at the beginning of the fiscal year and we’re able to do our scientific mission and continue to really carry out and support the stunning, breathtaking progress that’s going on in cancer. We are grateful to be able to continue our work and we recognize the disruption many of our colleagues in other agencies are facing.
We had a bit of concern about our ability to convene meetings that have to be published in the Federal Register. We think we’ve resolved that issue. We believe for present time, at least, we’ll be able to convene our relevant councils on time.
As the partial shutdown continues, going forward we will be placing a high level of scrutiny on some of our activities such as travel. I will be canceling my trips until the current situation is resolved and I will be asking the NCI staff to carefully consider planned travel and limit trips to those that are mission-critical and time-sensitive. I do not anticipate that will distract significantly from our scientific mission or our scientists’ ability to do their work.
Matthew Ong: You had a Town Hall today. What’s the news?
NS: As you know, we had a bit of truth-telling at the most recent joint National Cancer Advisory Board and Board of Scientific Advisors meeting about the state of the NCI budget. The budget is in many ways good—we certainly are not complaining about $180 million increase we got this year. There are some things, some trends within cancer research in general that are contingent on the NCI and that have led to moving funds around within the NCI. I wanted to better explain why we’re doing that.
Basically, the news is that because of the success of cancer research, we’re having so many people in our field and we’re getting so many great ideas. We’ve had this very sharp uptick in the number of grant applications we’re receiving for certain kinds of grants like the R01 award. Grants are up nearly 60 percent since 2009 and nearly 50 percent since 2013.
This is really strong increase, but it’s not been seen across the rest of the NIH. We think that’s because scientists are being drawn to cancer research by the scientific opportunity there. This is mirrored in pharma as they are devoting more resources to oncology and away from other therapeutic areas. I consider that good news. That’s a sign of a vibrant, healthy field where, new scientific ideas would be brought to bear.
But the problem you see right away is if your budget goes up 20 percent since 2009 and your grant applications go up 60 percent since 2009—you know this math—then, that’s going to lead paylines to go down despite the fact that we’re putting more and more money into the RPG pool every year.

In 2018, we had the biggest increase in the RPG pool since 2003 a $120 million-plus increase in the RPG pool. And this year, we hope to put an additional $100 million or more into the RPG pool, even though, as you know, the increase to our budget this year is roughly $180 million dollars, but $100 million of that is for the Moonshot. We will use the Moonshot funding for Moonshot purposes, so really, the increase to our general appropriation this year is $80 million, and we plan to put more than that into the RPG pool.
PG: What’s your goal for a success rate? I mean, you’re having a really good problem.
NS: I agree, Paul. And thank you for discussing it that way. Some people trying to get an R01 from the NCI, may not necessarily see it as a good problem, but I have to keep reminding people that in some ways, it is.
But I think paylines last year were 9 percent that translated into a 12 percent success rate. We think paylines and success rates will both go down slightly in 2019.
And I think if we didn’t make adjustments for fiscal year 2019—modest reductions to operating budgets for NCI divisions, reducing new, non-modular R01s by an additional two percent, and reducing many noncompeting grants by 3 percent—paylines would be lower still.
The adjustments we’re making adjustments allow NCI to put more money into the RPG pool and keep the payline as healthy as possible.
How are the “haircuts” –as Harold Varmus used to call them—for NCI’s budget proceeding? Have they been enacted?
NS: We are enacting them. I’m having discussions with all division directors about how they’re experiencing the cuts to their operating budget. Grants and salaries are exempted from the division cuts. So, the cuts to the actual divisions are smaller than you might imagine.
But the traditions at the NCI for the last few years have been for the budgets to go up. So, any cut at all is different from the status quo and it is causing some hardship. I think that no one’s enthusiastic about this, but I think everybody understands the issue. We’ve discussed these data about increasing grants internally and everyone agrees it’s a good problem, as we said, so, the NCI leaders agree that we want these grants coming in and we’d like people to send their ideas for funding.
We want to try to support them and really support innovation to the extent possible. And therefore, I think the purpose of the haircuts, as you called them, is well understood. And I think there’s buy-in on why we have to do this.


And also, of course, NCI has the largest intramural program at NIH, the last time I checked. So, there’s some room there, potentially.
NS: A criticism lobbed at the NCI from the extramural world sometimes is we don’t have the means to really evaluate ongoing programs and decrease them or terminate them when timely. These haircuts really forced the division chiefs to do that to some extent. I’m asking them to find the savings in their division and they are using this opportunity to really think about what their priorities are. So, in some ways it’s not unhealthy for an organization that can do things like this.

Are the percentages still the same? Five percent cuts to the NCI divisions, offices, and centers, and 3 percent cuts to the noncompeting awards?
NS: The three percent cut only applies to certain types of noncompeting, continuing awards. First off, let me say what we’re not cutting. We’re not cutting training grants, cancer center support grants, SBIRs, the Moonshot, and very specific small awards. But beyond that, all the other continuing RPGs are getting cut.
We decided to not to apply the cuts to cancer centers. The cancers centers are very good investments for the NCI. I’ve traveled to most of the cancer centers and I think for every dollar we spend there, they bring in—in some places it’s $5, in some places a lot more than that.
There are two main reasons why we decided to not to apply the cuts to cancer centers. The cancers centers are very good investments for the NCI. I’ve traveled to most of the cancer centers and I think for every dollar we spend there, they bring in—in some places it’s $5, in some places a lot more than that.
So, it is a highly leveraged funding. But the other reason and probably the more important one, frankly, is that [former NCI Director] Harold Varmus asked to refine to cancer center funding model.
There were years of discussion about this and we finally hit upon a plan that would involve increasing the funding of small centers. So that plan was to be implemented over five years as each of the existing centers come in for recompetition.
We’re committed to that plan and we’re only one year into it. This is a difficult funding model to implement and now that it has started, I don’t feel like it’s time to back away from it yet.
So, the cancer centers are a good investment and we’re going to continue to maintain our commitment to the funding model. Now I can’t say, if we were to have an even more difficult budget year next year that the cancer centers would always be off the table. But I think for this year, at least, we’ve preserved them.


How is NCI using this year’s Moonshot funds? At $400 million, it’s the largest authorization in the seven-year lifespan of the initiative.
NS: That’s right. First off, we are using the Moonshot funds for the purposes identified by the Blue Ribbon Panel. As you know, science evolves and some of the opportunities look a little different today than they did three or four years ago. For the most part we are trying to hew as closely as possible to the plan established by the vision identified by the Blue Ribbon Panel.
In other words, we’re sticking to the Moonshot goals. As you know, there’s flexibility in the funding that allows us to fully fund certain grants in the first year and we could carry over other funds as needed. And so that flexibility is really important this year, because $400 million is the high watermark, next year it goes to $200 million.
We have to plan for that so that we can experience that in a way that’s not fiscally irresponsible for the NCI. As you know, most of the awards, even though we do have the ability to pre-fund the grants to be funded over multiple years. And so, the things we award this year will have significant out-year costs, so we have to plan for that too.
Therefore, I would say that there will be a lot of new funding announcements this year along the lines of the Moonshot goals. You have the 10 recommendations from the Blue Ribbon Panel. But this will be the last big year for the Moonshot. Next year, we will have some monies for new initiatives.
This will not be the last year of new initiatives in the Moonshot, but it’ll be less because next year, the proportion of the budget consumed by our costs will be a lot higher. So, I’ve been telling people that are interested in getting funding that they carefully look at the RFAs on the Moonshot website, because there are several open now and there’ll be a lot of additional ones opening this year.
So, basically it sounds like this year’s Moonshot increase allows the institute to jumpstart funding for more projects and RFAs?
NS: That’s right. I think the 10 recommendations of the Blue Ribbon Panel are really exciting. There’s some great stuff in there and we have been working diligently on new concepts that will be published this year. The Blue Ribbon Panel’s vision was a good one and it allows us to really dedicate very targeted funding to important, mostly translational, initiatives. The idea is getting cutting-edge science into patient care as rapidly as possible.
In JAMA this week, you co-wrote a piece with Jim Doroshow in which you described the NCI approach to modernizing clinical trials. Could you recap the institute’s new approach to clinical trials? How is it being done and what might be the impact on the groups and the cancer centers?
NS: I think this is such an important topic. When I talk to colleagues at cancer centers, I hear a lot of frustration, but also just a lot of questions, a lot of lack of clarity.
It seems like people don’t understand what the NCI’s vision is for clinical trials. So, I think we have to admit, as a specialty, that clinical trials have really changed in oncology and that’s a good thing. It reflects scientific progress and better drugs for patients with less toxicity.
But it does mean we have to do things differently. And there are a lot of people who liked doing things the old way and so that’s a little uncomfortable for some of them and getting them to buy into these changes is really the NCI director’s job. We’re trying to make the point in a number of ways—different structures of trials, things like the NCI-MATCH trial or Lung-MAP, or the DART trial, it’s trying to not compete with industry but complement industry.
So, we have to accept that industry sponsors fund the majority of clinical trials in oncology in the United States. NCI has to really decide what are the kinds of trials that it should do with our considerable investment. We spend a lot of money on clinical trials, but that money should be complimentary to what is being spent by industry, not competitive.
So, I thought about a lot of what these trials are, things like deescalation studies and multimodality trials, and trials that combine different agents from multiple companies that are hard for them to do for a variety of reasons. And really that’s sort of the sweet spot for the NCI to be involved.
And then, as you know, we are putting more funding into clinical trials and we believe that the clinical trials infrastructure has been under-resourced and we are trying to address that issue. We were able to add $10 million to the National Clinical Trials Network, for example, last year. I hope to be able to do the same this year. I don’t know if that will be enough.
I hope even in future years we will be able to add more money to NCTN, but at least for the first two years, so far so good. We’re also adding new money, I hope this year, to the NCORP. The NCI Community Oncology Research Program has become a really important venue to do many trials that are a good fit for community oncology, and that, additionally needs more support.
What do we need to know about NCI’s efforts on Big Data over the past year? Are you also expanding the institute’s work on real-world evidence?
NS: It’s an area where there’s a lot going on and we have a lot of dynamic, interesting initiatives, but I think some of them are a little less visible, because they tend to be weedy infrastructure stuff like building a different computer architecture that makes the data much more useful.
I’m not sure people notice unless they are direct consumers of that data on a day-to-day basis. But we’ve begun to implement this cancer research data commons framework. So, we took the success of what started with The Cancer Genome Atlas and then became the Genomic Data Commons, and realized that single node can be replicated for other kinds of data—data commons for imaging and data commons for proteomic data, and data commons for a variety of other sorts of data.
And then, that can all be shared and linked through a common metadata data aggregator. We believe the framework for using this will be these cloud initiatives that the NCI supported over the last few years, the so-called cloud pilots that we now call the cloud resources, because we’ve been very successful and we continue to support that.
So, we have a pretty clear vision for how we’re going to get more data to our academic investigators as quickly as possible, but the details of each node matter, like how do you get the clinical annotation for the genomic data—that turns out to be an authority problem that we are talking with industry and academic partners about how to do that better. We have a real focus on working with the FDA and we’ve had a lot of lively, ongoing discussions with Sean Khozin and colleagues at the FDA about how the NCI can use some of their pristine and wonderful trials data for our purposes. I think the FDA’s enthusiastic about that.
I also believe, with Amy Abernethy coming to be the deputy commissioner [at FDA]—she’s a longtime friend of mine, but she’s also somebody who really understands Big Data at a very sophisticated level, given her background in Flatiron—I think will be a real champion for data usage between the FDA and NCI.
And then, lastly, and perhaps most importantly in data, I’m about to hire a new director for the Center for Biomedical Informatics and Information Technology. Although the title sounds a little weedy, I really envision that person being the visionary, data strategist for the National Cancer Institute, and so, that person will be very important to figure out where we need to be in 20 years.
Can you tell us who that is?
NS: I’ve been interviewing candidates. I will say the application pool is very strong. The search committee was led by Stephen Chanock and they’ve produced a great roster for me to interview. I’m midway through the interviews and I’m sure we’re going to find somebody great.
You’ve laid out your vision for the institute since you’ve become director—how are these priorities coming along? How’s your checklist looking?
NS: You know, some checks are bigger than others, pun intended. I have been encouraged. I feel like the extramural community has bought into these key focus areas (big data, clinical trials, workforce development, and basic science).
What I’m finding is a lot of people saying, “How can we help? And if you’re interested in Big Data, you should do this.” For example, I was at the Broad Institute and they have a lot of great ideas on how the NCI could do data differently. And so, we really need to take advice from the extramural community, be educated by them.
I’ve become very fond lately of this Harry Truman quote: “Doing the right thing is easy. Knowing what the right thing to do is hard.” We have a lot of laudable things we want to do, but exactly how you’re making them happen mechanically can be challenging.
I also feel like the intramural program is very strong and continues to advance these sorts of scientific areas where the intramural program is really well suited to do that. I feel like my efforts to get out within the intramural program and meet people and have a lab on campus and to really understand what’s causing difficulties for the scientists, has gone over well.
And then, I’m also now becoming more involved with the NIH Clinical Center which has a lingering set of issues. As you may know, the NIH suffered a tremendous loss late last year when Steve Katz passed away. Steve was the director of NIH’s National Institute of Arthritis and Musculoskeletal and Skin Diseases and served as the chair of NIH’s Clinical Center Governing Board for many years. [NIH Director] Francis Collins has asked me to take over that chair role for the clinical center because of my passion on this topic, so I hope to continue to support the intramural program and the clinical center as much as possible. And I think that has been very appreciated in my first year at the NCI.
Do you have any new plans or goals for NCI in the new calendar year? Anything you didn’t see before that you see now?
NS: I think they will look new to some people, because they will be the extension of the things that we’ve said from day one we’re trying to do, but I think we’ll now start to see some of those things happen.
We’ve discussed a lot about new funding announcements, and for the Moonshot, some of those will actually come to fruition this year. And as you’ve probably followed, a few of those topics were particular challenging if you had a lot of feedback in discussion with the NCAB to get those cleared and the BSA to get this cleared.
I think now I’m very excited about some of these things that will be coming out this year. I think the continued focus on clinical trials is starting to play positively and then, we have some new stuff in the works that hopefully we’ll be able to talk about more as they come to pass. But they will be, in a way, hopefully surprising new visions, but also hopefully unsurprising and is exactly in line with the things we said we will do.
Is there anything we missed?
NS: The state of the NCI is good. As we’ve discussed, in 2019, NCI will increase support for cancer research through RPGs, we’ll stay true to the vision of the Cancer Moonshot, and we’ll continue to support early-stage investigators and cancer centers.
Any downward trend in the payline is concerning, but it has to be seen as a good problem. The field is vibrant, and competition is good for science. Good cancer science will ultimately benefit patients.
I don’t know if you saw Gideon Blumenthal and Rick Pazdur’s article in Nature Reviews Oncology that came out Tuesday, but it shows all these drugs approved. It’s just remarkable and that doesn’t happen by accident. That certainly doesn’t happen solely by industry.
If you look at those drugs, many of them are the result of long, detailed, basic science and clinical trials funded by the NCI. And so, I think we can claim a lot of credit for that success there.










